Recreating cardiovascular disease using soft robotics
Published in Bioengineering & Biotechnology

The development of medical devices requires testing platforms that recreate the disease being treated. The closer the multifaceted aspects of the disease are simulated by such platforms, the more representative are the tests conducted. Higher fidelity testing helps to achieve the overarching goal of facilitating the development of effective medical devices and enhancing the impact that they can have on patients’ lives. For a number of cardiovascular conditions, it’s particularly crucial to recreate both the biomechanical and haemodynamic aspects of disease. In a collaborative effort between the Massachusetts Institute of Technology and Cleveland Clinic, we developed an implantable soft robotic aortic device that simulates, with high fidelity, the kinematics of the aortic valve and the haemodynamics associated with aortic stenosis – the most common valvular heart disease in the western world.
In physiology, the aortic valve regulates blood flow from the heart to other organs of the body. When the mobility of the valve leaflets is reduced, for example because of calcification processes or birth defects, the orifice of the valve may become smaller. This condition, known as aortic stenosis, induces changes in blood flow through the valve, often leading to secondary heart disease and even heart failure. If untreated, aortic stenosis may kill up to one in two individuals within two years of diagnosis. Replacement or surgical repair of the aortic valve are the only treatments for this disease, which is why continuous efforts are being made by laboratories at academic institutions and R&D departments around the world to augment the efficacy of these interventions and improve the design of new prosthetic valves. Most current animal models of aortic stenosis fail to recreate the complex 3D flow patterns observed in aortic stenosis and their limited control prevents them from recapitulating the haemodynamics of congenital aortic valve defects, which often accelerate the onset and progression of aortic stenosis.
Led by PhD Candidate Luca Rosalia and postdoctoral researcher Caglar Ozturk, our work brings together the expertise in soft robotic design of Ellen T. Roche, Professor at the Massachusetts Institute of Technology, and in cardiovascular imaging of Prof. Christopher T. Nguyen, Director of the MRI Research Center for Advanced Imaging and Advanced Simulation at Cleveland Clinic, who jointly supervised this project. We realised that by utilising materials with mechanical properties similar to those of biological tissues, soft robotics could help us simulate the biomechanical function of the aortic valve and mimic changes associated with disease more closely than current models. Therefore, we engineered a bioinspired soft robotic aortic sleeve that could recreate the complex motion dynamics of a stenotic aortic valve and leveraged Magnetic Resonance Imaging (MRI) to demonstrate the enhanced mimicry of flow patterns of aortic stenosis achieved by our platform. Inspired by the anatomy of the tri-leaflet aortic valve, our design consists of three expanding pockets, which can be pressurised to simulate stenosis. We heat-sealed these pockets to an inelastic fabric sheet to control the direction of motion in response to pressure. The soft robotic sleeve can be operated under two distinct conditions: quasi-static volume control to recreate the progressive nature of the disease, and dynamic pressure control to accurately mimic the motion of the valve leaflets. Further, it can be reconfigured to recreate the morphology of aortic stenosis and congenital defects, such as unicommissural and bicommissural disease, as shown in Figure 1a.
Combining in vitro and computational approaches, we studied the biomimetic soft robotic sleeve's mechanical behaviour and its impact on blood flows and pressures in simulated models. Computational methods involved lumped-parameter, finite-element, and computational fluid dynamics platforms. Using these models, we could predict the haemodynamic changes induced by actuation of the biomimetic soft robotic aortic sleeve for aortic stenosis and congenital defects. In addition, we were able to visualise the aortic flow and velocity vector fields resulting from actuation of our sleeve through MRI techniques. Following in silico and in vitro characterisation, we then demonstrated the functioning of the soft robotic sleeve in a porcine model. We used MRI, echocardiography, and catheterisation of the pig heart (Figure 1b) to demonstrate that our simulator could accurately reproduce the complex blood flow patterns associated with aortic stenosis and congenital defects in a wide range of clinical scenarios.
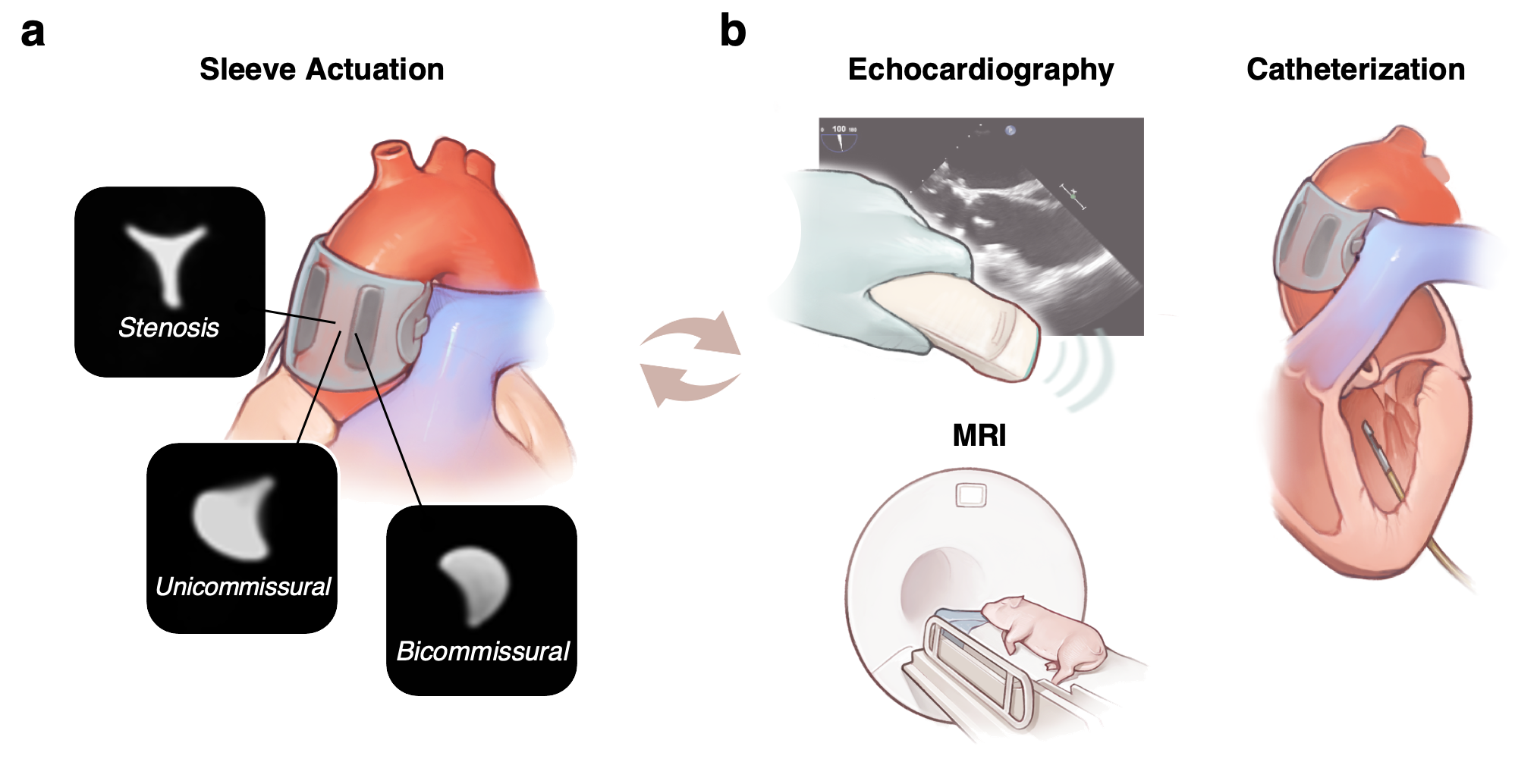
Figure 1 – Biomimetic soft robotic aortic sleeve modalities and characterisation. a, Stenosis (tricuspid), unicommissural, and bicommissural morphologies recreated via customisation of actuation modality of the sleeve and visualised on MRI. b, Characterisation of the sleeve in a porcine model through echocardiography, MRI, and heart catheterisation.
Through this work, we could show that recreating the motion dynamics of stenotic aortic valves and the haemodynamics associated with mild, moderate, and severe forms of disease could be relevant for preclinical and translational studies of human physiology and disease. The development of an enhanced platform that can recreate changes in cardiac and valvular biomechanics and haemodynamics may have profound implications for the medical device industry, as it is poised to support translational efforts in the development of next-generation medical devices for the treatment of aortic stenosis. Beyond the cardiovascular field, our work suggests that high-fidelity soft robotics-based models could be used to simulate other conditions for a broad range of biomedical applications transforming the future of device development and testing, and ultimately improving the management, quality of life, and survival of millions of patients affected by cardiovascular and other disease.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biosensing
Publishing Model: Hybrid
Deadline: Mar 26, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in