Sex-specific lipidome changes associated with diabetes
Published in Cell & Molecular Biology and General & Internal Medicine

The research involved 360 participants: 91 with T1D, 91 with T2D, 74 prediabetic individuals, and 104 controls. All participants were free from previous cardiovascular or chronic kidney disease. The study utilized untargeted lipidomic analysis through Ultra-high performance liquid chromatography-electrospray ionization mass spectrometry (UHPLC-ESI-MS) to assess lipid profiles. Multiple linear regression models were employed to examine the associations of different lipid classes with diabetes, while considering both sex-specific differences and glycemic states.
Study findings
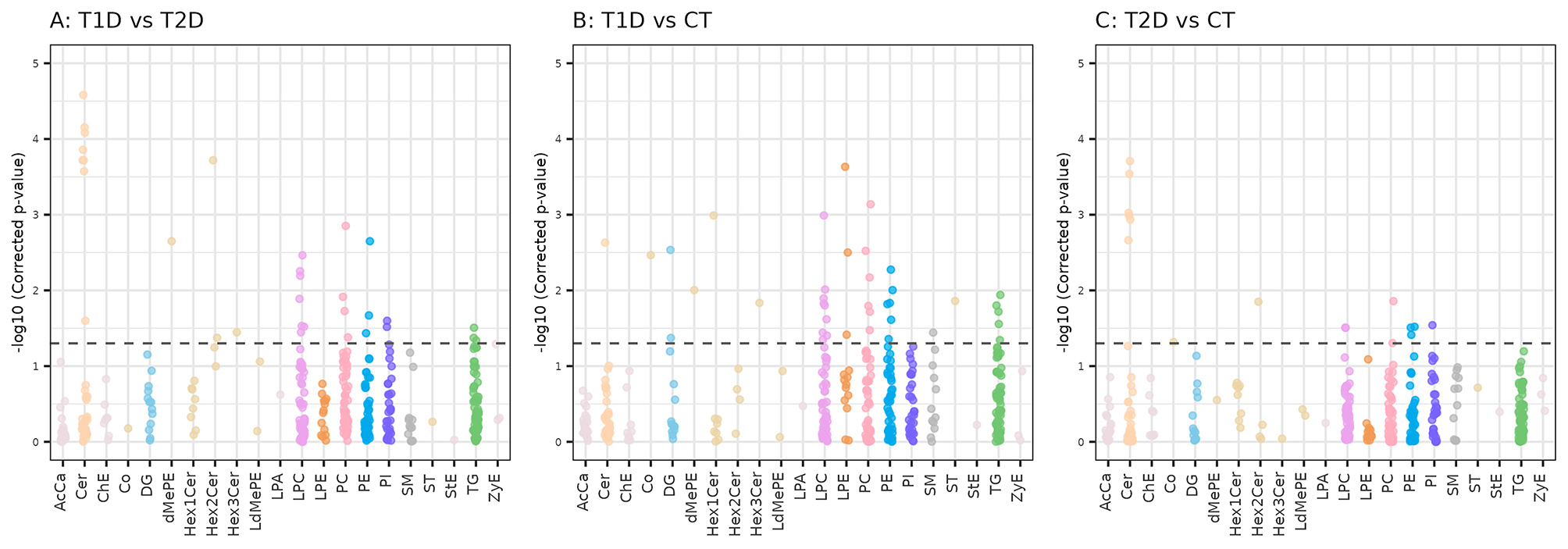
The study identified 54 unique lipid subspecies from 15 unique lipid classes, highlighting a diverse lipidomic profile associated with diabetes.
Lysophosphatidylcholines (LPC) and ceramides showed opposite effects in subjects with T1D and subjects with T2D. Lysophosphatidylcholines were significantly up-regulated in T1D participants but down-regulated in those with T2D. This suggests that LPC metabolism differs markedly between these two types of diabetes. This increase in LPC levels may correlate with increased LCAT activity, which is involved in lipid metabolism, particularly the synthesis of phosphocholine and cholesteryl esters from LPCs and phosphatidylcholine.
Ceramides displayed the opposite trend compared to LPCs; they were found to be up-regulated in subjects with T2D while showing down-regulation in T1D. Variants such as 1-deoxyceramides exhibited a significant increase in T2D, marking them as important indicators of the disease. These findings indicate a clear alteration in ceramide metabolism in type 2 diabetes that may influence insulin signaling pathways, potentially worsening insulin resistance.
Phosphatidylcholines (PC) were predominantly down-regulated in the subjects with type 1 diabetes; this may have implications for membrane integrity and function. This down-regulation contributes to the overall altered lipid profile observed in T1D.
Interestingly, the study identified sex-specific lipidomic differences. For instance, lipid alterations were notably more pronounced in men regarding certain ceramides. Specifically, specific ceramide molecules were significantly decreased only in men with T1D. Additionally, higher levels of phosphatidylcholines were present in normoglycemic women than in men. These findings point to the role of hormonal factors in determining sex-specific lipid metabolism differences.
We also analyzed possible associations of lipid classes with the glycemic state, i.e. normoglycemia, prediabetes and type 2 diabetes. A gradual increase in 1-deoxyceramides was seen progressing from normoglycemia through prediabetes to T2D, indicating these lipid species might serve as potential early biomarkers for diabetes progression.
Clinical and research implications
The findings underscore a substantial disruption in lipid metabolism for both types of diabetes, indicating that further investigation into lipidomic profiles may yield critical insights into diabetes pathophysiology. This could lead to enhanced understanding of the disease mechanisms and the identification of novel biomarkers for diabetes. Furthermore, from the clinical point of view, our results suggest the need for sex-specific strategies in diabetes management. This personalized approach to treatment could enhance patient outcomes and open avenues for tailored therapeutic interventions. Moreover, the future potential introduction of lipidomic profiling characterization into routine clinical assessments could contribute to more precise management of diabetes and its complications.
Overall, this study contributes relevant knowledge to the field of diabetes research, emphasizing the potential of lipidomics in personalizing treatment strategies while highlighting the importance of sex differences in metabolic responses.
Follow the Topic
-
Cardiovascular Diabetology

This journal considers manuscripts on all aspects of the diabetes/cardiovascular interrelationship and the metabolic syndrome; this includes clinical, genetic, experimental, pharmacological, epidemiological and molecular biology research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Organoids as emerging models in diabetes and cardiovascular research
Organoids—three-dimensional structures derived from stem cells—are transforming biomedical research by modeling key aspects of human physiology and disease. By replicating native tissue architecture, cellular heterogeneity, and functional behavior, they provide human-relevant systems that address limitations inherent to conventional in-vitro and animal models.
Diabetes and cardiovascular disease are deeply interconnected conditions, characterized by shared, multi-organ pathophysiology. Organoid technologies offer unique opportunities to dissect disease mechanisms, evaluate therapeutic strategies, and develop personalized, physiologically relevant models. These systems enable the investigation of cardiometabolic processes in platforms that better reflect the complexity and progression of human disease.
Cardiovascular Diabetology welcomes original research articles, reviews, and meta-analyses for this Collection, which aims to highlight the use of organoid technologies in advancing our understanding of cardiovascular complications associated with diabetes.
Areas of interest include, but are not limited to:
- Organoid models of diabetic cardiomyopathy and heart failure
- Matrigel alternatives for organoid development
- Cell-cell and extracellular matrix interactions in organoids
- Organoid-based drug testing for cardiovascular diseases
- Organoid-on-chip systems for tissue crosstalk and perfusion3D bioprinting and tissue engineering for cardiovascular organoids
- Artificial intelligence–driven analysis of organoid function and phenotypes
- Organoid models of gestational diabetes–induced congenital heart disease
- Functional genomics using CRISPR in cardiovascular organoids
- Single-cell and spatial omics to map disease states in organoids
- Co-culture systems of vascular and pancreatic organoids to study metabolic-vascular crosstalk
- Organoid-based screening platforms for anti-diabetic and cardioprotective drugs
Submissions that contribute to conceptual clarity (e.g., distinctions between organoids and spheroids), incorporate multi-organ or metabolic system perspectives, or connect technological development with clinical or translational insights are especially welcome.
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jul 07, 2026
Cardiometabolic and Hepatic Interconnections: From Mechanisms to Clinical Implications
Cardiometabolic and liver diseases are no longer viewed as isolated entities. Growing evidence shows that hepatic and cardiovascular dysfunctions are tightly interconnected through shared metabolic, inflammatory, hemodynamic, and hormonal pathways. This crosstalk shapes disease trajectories and opens opportunities for integrated diagnostics and therapies.
Key interconnections include:
- Insulin resistance. The liver is pivotal for glucose and lipid homeostasis. Hepatic insulin resistance drives excess glucose production and dyslipidemia, contributing to the cardiovascular–kidney–metabolic (CKM) syndrome.
- Metabolic dysfunction–associated steatotic liver disease (MASLD). Highly prevalent in obesity and metabolic syndrome, and especially common in type 2 diabetes (T2D), which bears the highest MASLD burden. Progression to steatohepatitis or fibrosis markedly increases atherosclerotic risk.
- Liver-derived factors. Hepatokines (e.g., FGF21) and extracellular vesicles influence cardiac tissue, vascular tone, and systemic metabolism, potentially amplifying inflammation, oxidative stress, and lipotoxicity across organs.
- Systemic inflammation and lipotoxicity. Visceral adipose tissue and the liver release inflammatory cytokines and triglyceride-rich lipids, promoting endothelial dysfunction and perpetuating metabolic disturbances.
- Dyslipidemia. Elevated triglycerides and LDL, with reduced HDL, accelerate atherogenesis and cardiovascular risk.
- Hemodynamic and metabolic stress. Heart failure can cause hepatic congestion and hypoperfusion, while advanced liver disease can precipitate cirrhotic cardiomyopathy and arrhythmias, even in the absence of prior heart failure.
In summary, hepatic and cardiometabolic systems are functionally and pathologically intertwined: liver dysfunction worsens cardiovascular and metabolic health, and cardiometabolic disturbances accelerate hepatic pathology.
This Collection welcomes original research articles, reviews, and meta-analyses focused on this reciprocal relationship. Given the high prevalence of MASLD in T2D, we especially encourage submissions at the T2D–MASLD–cardiovascular interface. Addressing hepatic and cardiometabolic health in parallel is essential for effective risk reduction and patient care.
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this Collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jul 15, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in