Simultaneous aerobic-anaerobic respiration: A microbial tale from the mud
Published in Microbiology

It all began in the mud
The Center for Electromicrobiology and Microbial Electricity research groups have long been fascinated by microbial electricity in sediments, especially the enigmatic cable bacteria. These strange filamentous microbes live in aquatic sediments, conducting electrons over centimetre distances between the sulphide-rich deeper sediment and the oxygenated surface layer. Observing cable bacteria under the microscope, we noticed dense clusters – or “flocks” – of other bacteria crowding near them. We began to wonder: could these flocking microbes be interacting with cable bacteria? Could they be electroactive too? We took on the challenge, and a side project was born.
An interesting yellow colony
Jamie isolated several colonies from the oxic–anoxic interface of sediments containing cable bacteria. Kartik screened them for electroactivity, and one yellow colony stood out: a Gram-positive bacterium we later named Microbacterium deferre A1-JKT. It originated from the oxic-anoxic interface, a microscale environment known for its redox complexity. Here, oxygen levels fluctuate due to diffusion and rapid microbial respiration, and iron is continuously oxidised, offering a dynamic range of potential electron acceptors.
Next, with the classical electrochemical workstation (and breaking a few electrodes along the way), Kartik's electromicrobiological studies finally got a result that stopped us in our tracks: M. deferre A1-JKT could respire using an electrode, secreting flavins as redox shuttles. It even maintained electroactivity across different pH levels.
We were particularly struck by how well this physiology fit with the natural habitat from which M. deferre A1-JKT was isolated. In sediments colonized by cable bacteria, rapid shifts in oxygen and pH occur due to cable bacteria actively transporting electrons over centimetre distances. To survive here, a microbe needs to readily adapt to the changing conditions, and must be able to switch between aerobic and anaerobic modes of respiration. M. deferre A1-JKT appeared to do just that.
A gut feeling that gave our breakthrough
As we compiled our results, Kartik had a gut feeling: what if M. deferre A1-JKT could respire simultaneously on Fe(III) and oxygen, both of which fluctuate in its natural habitat? After discussing the idea, we designed a follow-up experiment to test this possibility. The experimental design itself was quite simple. In a Hungate tube, we inoculated M. deferre A1-JKT and provided soluble iron and oxygen as the electron acceptors. With an oxygen spot sensor, we measured the consumption of oxygen, while the ferrozine assay was performed to estimate iron reduction. The outcome surprised all of us: oxygen levels dropped, and Fe(II) levels rose—simultaneously (Figure 1). It really was true – iron reduction and oxygen respiration were occurring together! The result seemed too extraordinary to be real, so we repeated the experiment, re-checked all controls, and confirmed it: aerobic and anaerobic respiration were occurring at the same time. Since we stirred the culture to avoid biofilms, the activity came from planktonic cells - suggesting this was happening at the single-cell level.
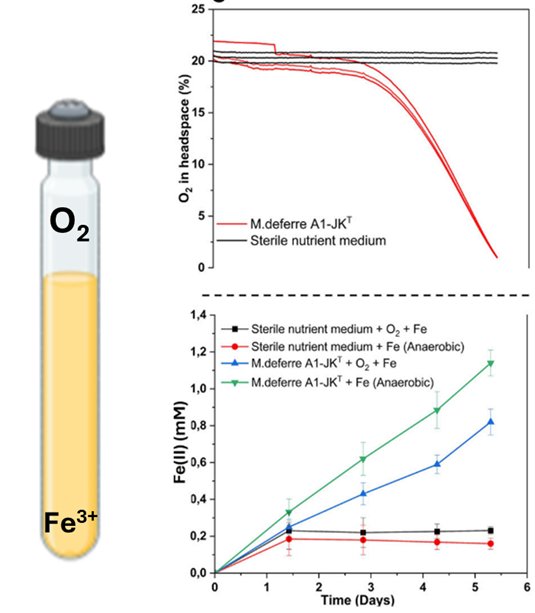
Figure 1 (from the manuscript): M.deferre A1-JKT cells were simultaneously able to reduce Fe(III) and oxygen provided as electron acceptors
Unexpected EET insights
Another surprise came from the extracellular electron transfer (EET) mechanism. Despite lacking the canonical flavin-based EET (FLEET) genes—pplA, eetA, eetB—M. deferre A1-JKT secreted riboflavin as a redox shuttle. Genomic analysis pointed toward a hybrid EET system: flavin reductase FmnA and cytochrome FccA, alongside a complete flavin biosynthesis pathway (RibBADE1E2). This system seemed to be perfect for metabolic flexibility in fluctuating redox conditions at the oxic-anoxic interface.
Good friends, good research
Working on this project was scientifically enriching and personally rewarding, bringing friends closer together. It brought together diverse expertise – electrochemistry, microbiology, genomics – and, more importantly, a nice team consisting entirely of non-tenured early career researchers. Jamie’s genomic sleuthing helped uncover the hybrid EET system; Leonid brought precision to the electrochemical work; and Naja, a Bachelor’s student, added fresh energy and critical lab assistance that helped us move quickly.
Beyond the team effort, this discovery has broader implications. The ability of M. deferre A1-JKT to perform simultaneous oxygen and iron reduction reveals an unusual metabolic flexibility that likely offers a selective advantage in dynamic environments. It suggests a role for EET beyond its traditionally understood function of supporting anaerobic respiration. In this case, EET may serve as a mechanism to offload excess electrons for aerobic respiration, possibly preventing intracellular redox stress under fluctuating oxygen and iron conditions.
These findings also raise intriguing questions about the early evolution of microbial metbolism. Could the mechanisms of simultaneous aerobic and anaerobic respiration have ancient roots, dating back to the time when Earth’s atmosphere was just beginning to oxygenate during the Great Oxidation Event? Ultimately, this study shows that some microbes don’t just “switch” between metabolic modes—they combine them.
Sometimes, paradigm-shifting discoveries begin not with a grand project, but with a hunch, a side question, or an unexpected observation while chasing another story. This one started with cable bacteria and enthusiastic friends. It ended with an interesting yellow colony that challenges how we think about microbial respiration—and reminds us that nature still holds many surprises.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in