The Ghost of Obesity Past : How Adipose Tissue Remembers Obesity
Published in Cell & Molecular Biology, Biomedical Research, and General & Internal Medicine

Explore the Research
Nature - Not Found
Sorry, the page you requested is unavailable. The link you requested might be broken, or no longer exist.
Read the Paper
https://www.nature.com/articles/s41586-024-08165-7
How It All Began
Obesity is a chronic condition with significant metabolic consequences, strongly linked to various metabolic and cardiovascular diseases. A well-documented observation is that the body tends to defend increased body weight, making weight loss and maintenance notoriously challenging. This could be due to a type of "metabolic memory," where the body remembers and strives to return to its former state of obesity. This notion has been explored in the context of improving glycemic control in diabetes, where metabolic improvements can have lasting effects, even if control is lost again.
Could epigenetic changes at the cellular level drive this metabolic memory in obesity? One metabolic organ heavily impacted by both obesity and weight loss is adipose tissue. Adipocytes, with a remarkable lifespan of around 10 years, do not divide, making them an ideal model for studying epigenetic memory. Additionally, adipose tissue is easily accessible for human biopsies, allowing for longitudinal studies—something not feasible for most other organs.
Our study focused on three main areas:
- The cellular remodeling of adipose tissue after weight loss.
- Investigating transcriptional memory across all adipose tissue cell types using single-nucleus RNA sequencing (snRNAseq).
- Using mouse models to analyze the epigenetic changes in adipocytes and assess their retention post-obesity.
What we found
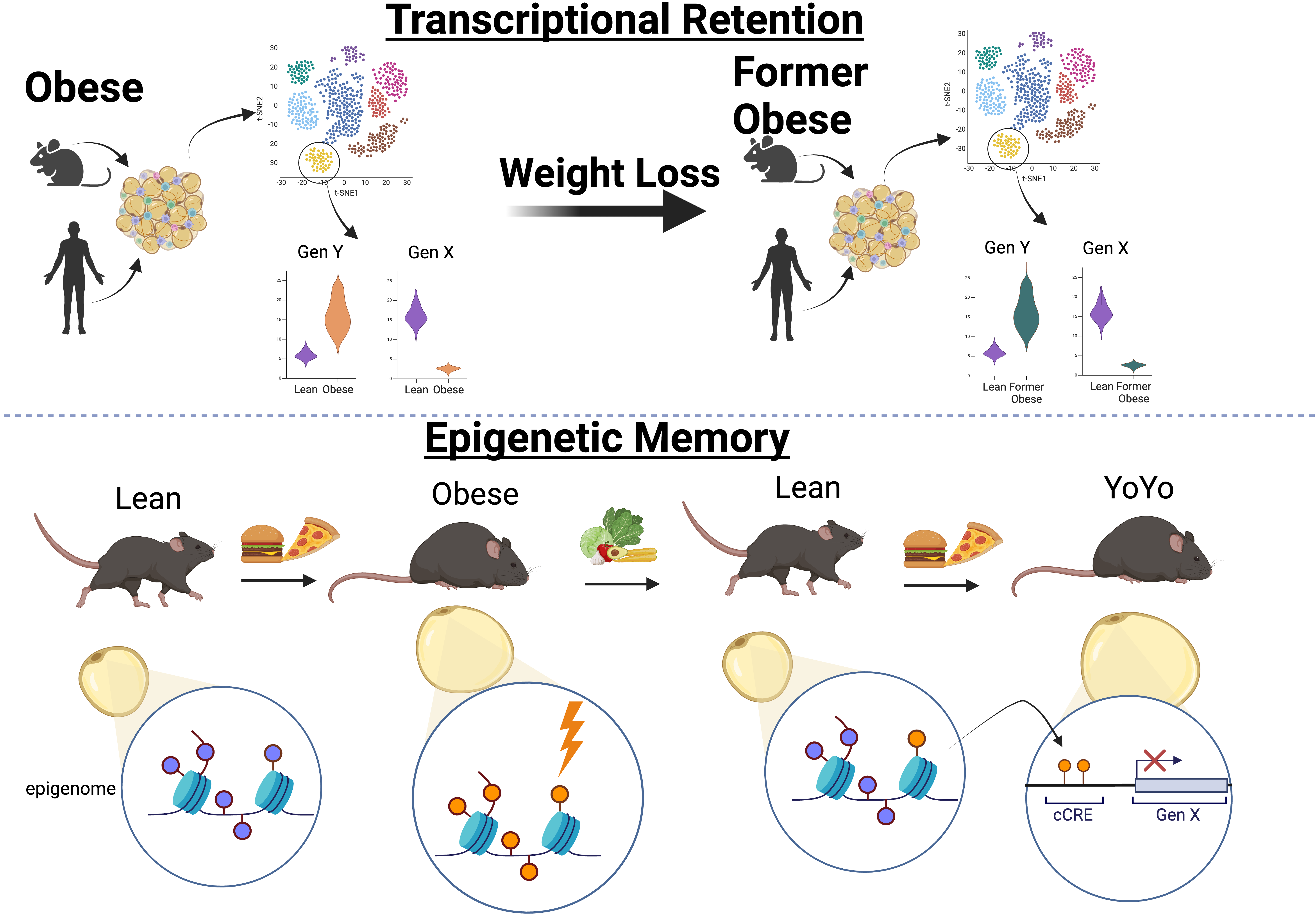
Unsurprisingly, we found differences in gene expression between cells from obese and lean individuals. However, the most striking discovery was that many of these differences persisted even after significant weight loss. This phenomenon was especially prominent in adipocytes, endothelial cells, and precursor cells, suggesting that these cells retain a transcriptional memory of obesity that remains long after weight reduction.
However, given the inherent limitations of human studies, such as genetics, nutritional status, the environment etc., we extended our analysis to mice. We induced obesity in mice through a high-fat diet (HFD) and then reversed it by switching them back to a standard chow diet. Unlike most dieting humans, mice achieved rapid weight loss, reaching body weights comparable to age-matched controls within 4-8 weeks. They exhibited normal metabolic function, including liver fat clearance, normalised insulin and leptin levels, and showing a near-complete restoration of energy expenditure.
Despite these apparent recoveries, the snRNAseq analysis of their adipose tissue revealed that adipocytes, endothelial cells, and precursors retained a transcriptional memory of obesity, consistent with our human data. This finding confirmed that metabolic memory is not exclusive to humans.
Why It Matters
This is one of the first studies to demonstrate that specific cell types can retain an epigenetic memory of a prior metabolic state. This finding has profound implications for our understanding of obesity, weight loss, and weight maintenance. On a societal level, this could offer some solace to individuals struggling with obesity, as it suggests that the difficulty in maintaining weight loss may not be due solely to a lack of willpower or motivation, but rather to a deeper cellular memory that actively resists change.
Moreover, if such epigenetic memories are found in other cell types, such as neurons, or in contexts like addiction, it could open new avenues for therapeutic interventions. Understanding and potentially reversing these epigenetic changes could have far-reaching implications for treating chronic conditions associated with metabolic memory.
What’s Next?
The question remains: why isn’t this epigenetic memory erased over time? Could it be because adipocytes do not divide? Or perhaps other cell types are similarly affected? Could it be that we merely need to stay lean long enough for the memory to vanish? The next steps will involve developing tools to precisely modify the epigenome at hundreds of loci to test whether altering these marks can reverse the obesogenic memory and its associated phenotypes. Additionally, it will be crucial to investigate whether long-lived cells, such as neurons in the hypothalamus, also retain an epigenetic memory of obesity.
The poster image was created with ChatGPT 4o.
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in