The Soluble Paradox: A Life Beneath the Surface
Published in Chemistry, Ecology & Evolution, and Neuroscience
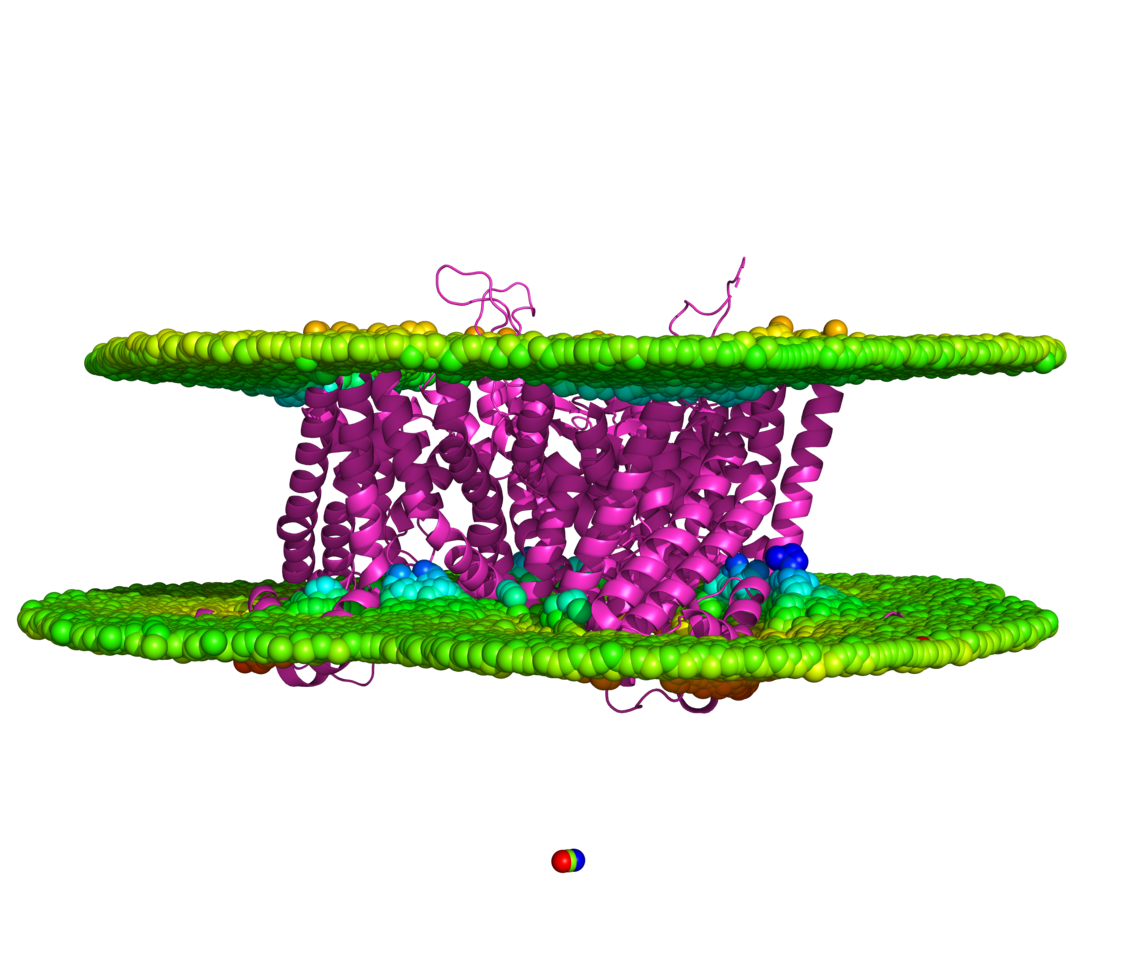
Every scientist has a molecule that traps a part of them, and these transporters were mine. They live inside the membrane, exiled from the clarity of water, too hydrophobic to touch, too essential to ignore. Their mystery was always protected by the same thing that made them indispensable: the lipid barrier that both shelters and silences them. I was told, more times than I care to count, that we could never truly see them as they are. That to extract them was to destroy them. That detergents and tricks were the price of knowledge.
But something in me refused that kind of obedience. There had to be another way
That’s how the QTY code entered my story, not as a tool, but as an act of defiance. Replace the hydrophobes L, I, V, F; with their hydrophilic reflections Q, T, Y. It was so simple it felt almost poetic: waiting for someone reckless enough to listen.
And so I listened.
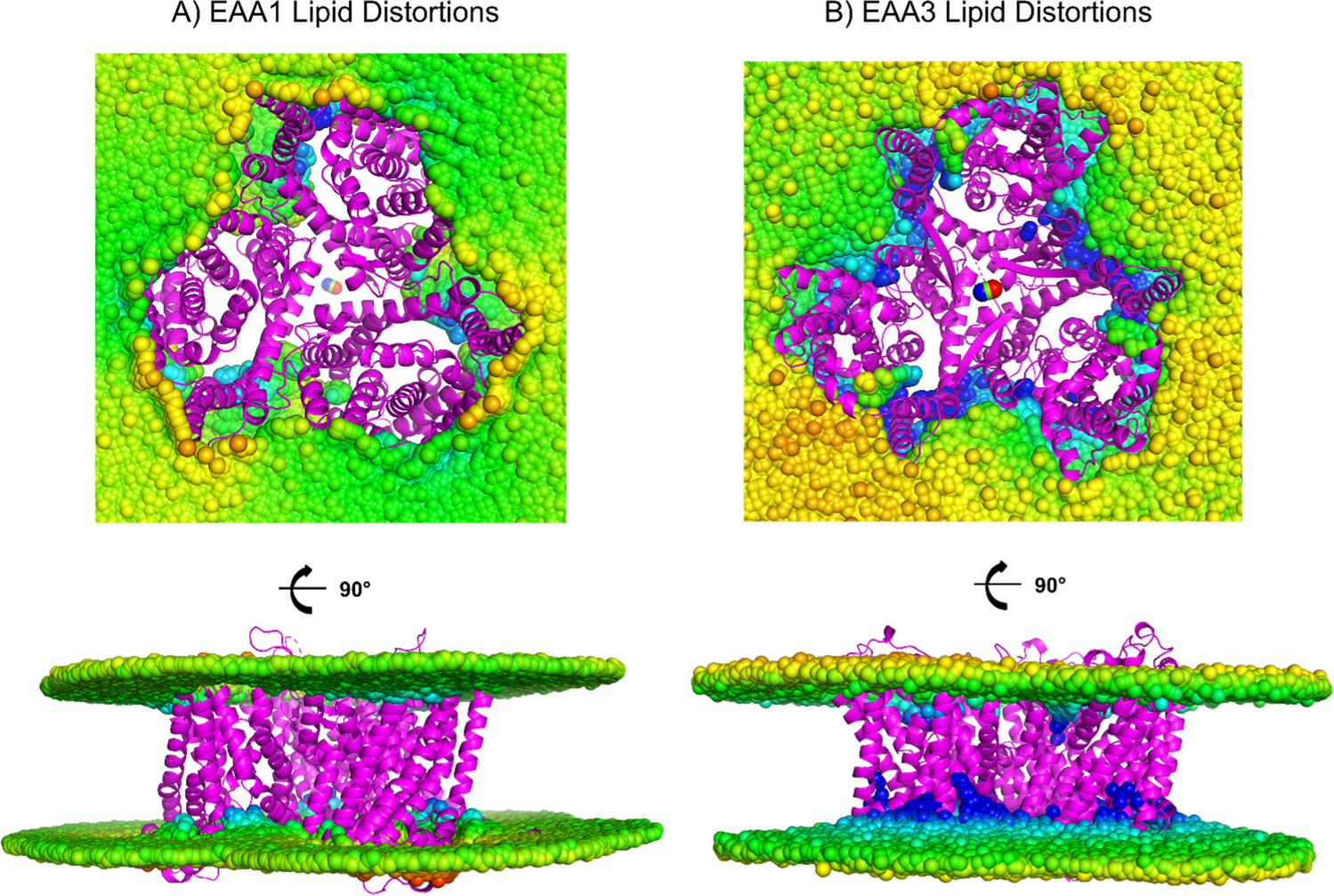
When I built the water-soluble variants (EAA1QTY, EAA2QTY, EAA3QTY) I was building not just proteins, but questions.
The first time I watched the molecular dynamics simulations unfold, I braced for collapse. I expected the structures to unravel, the helices to fall apart in the open water. Instead, they moved. They remembered their old choreography (Karagöl et al. 2024a, Karagöl et al. 2025).
I remember the data (correlation coefficients, RMSD values) but I also remember the feeling. These weren’t random substitutions. As if nature had already rehearsed this transformation long before we named it “QTY”.
To see a transporter, stripped of its hydrophobic armor, still fold and move with grace. It reminded me that identity, whether molecular or human, is not bound by environment. We adapt. We remember.
Science, at its most honest, is not about control. It’s about surrender: to uncertainty, to complexity, to beauty you cannot measure. I learned that even when we replace half of a protein’s heart, it can still find its rhythm (Karagöl et al. 2024b). They remind me that the line between the natural and the designed is thinner than we ever dared to admit.
The insoluble became soluble.
And I, somewhere along the way, did too.
Follow the Topic
-
Pharmaceutical Research

Pharmaceutical Research is an official journal of the American Association of Pharmaceutical Scientists, covering innovative research in drug discovery, development, evaluation, and regulatory approval.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in