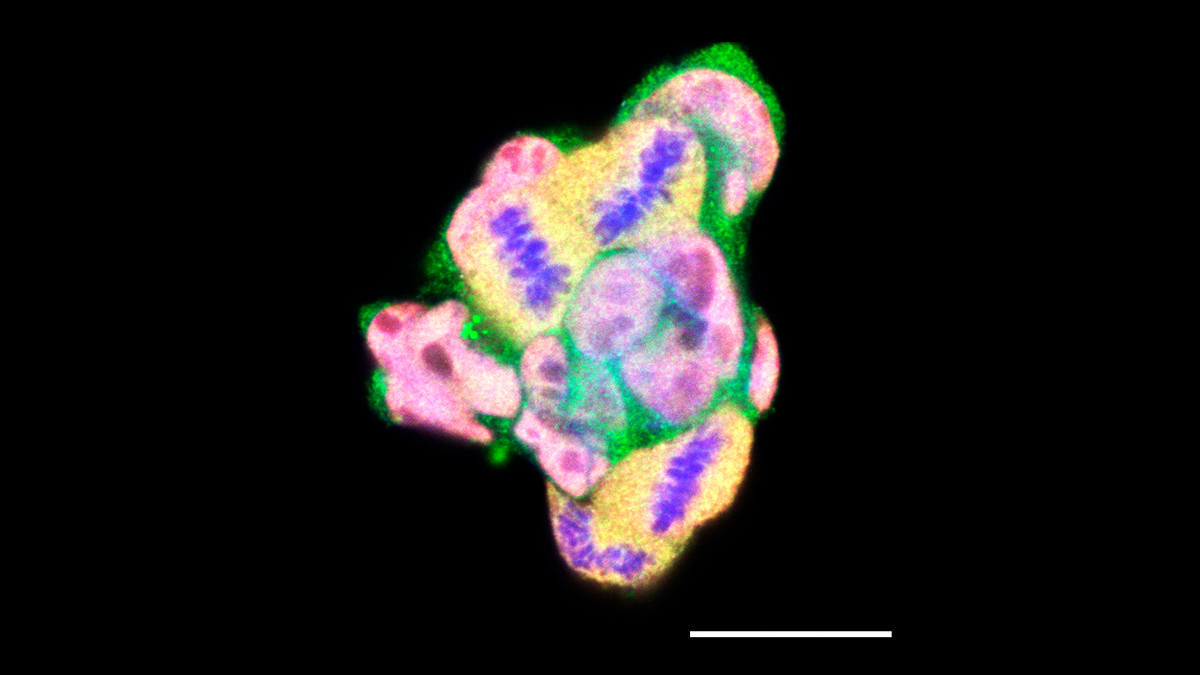
Our scientific journey began with a simple question about a protein called Stip1, which we had initially discovered as a binding partner of the prion protein. While the prion protein was our lab's main focus, we grew curious: what was Stip1’s own role in the body?
To find out, we collaborated with other researchers to generate a mouse lacking the Stip1 gene, a knockout model. The result surprised us: embryos without Stip1 simply could not survive. It was clear that Stip1 was not merely important, it was absolutely essential for early mammalian life.
While Sti1 has been described as a co-chaperone for HSP70 and HSP90, being important for cell protein homeostasis, or proteostasis, this was the first time it was associated with mammalian development (Beraldo et al., 2013). This important discovery led us to a new question: what exactly was STIP1 doing in early development?
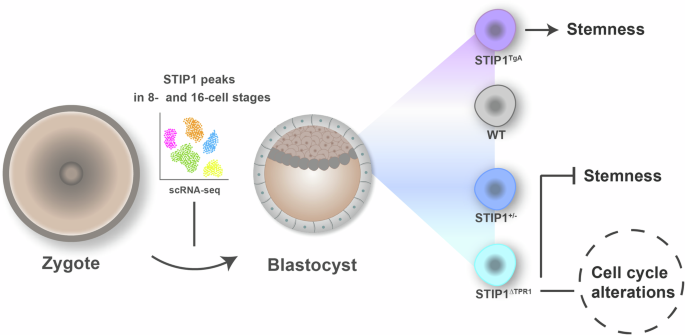
To answer this, we turned to mouse embryonic stem cells (mESCs), versatile cells capable of forming any cell type in the body. I traveled from Brazil to the lab of our collaborator, Dr. Marco Prado in the University of Western Ontario (Canada), to derive mESC lines (Figure) from genetically modified mice expressing different levels of STIP1: some low, some high, and some expressing a truncated form of the protein.
When I returned to my lab in the University of Sao Paulo, my team and I dove into the research, and what we found was striking.
Transcriptomic data suggested that Stip1 expression was tightly controlled in mouse and human embryos, and many genes important for embryonic development were significantly correlated with Stip1 expression. Moreover, cells with depleted Stip1 levels struggled to proliferate, showed reduced expression of key pluripotency markers, and frequently underwent cell death (apoptosis) along with increased genomic instability. In contrast, cells overexpressing Stip1 displayed enhanced pluripotency markers expression, accelerated proliferation, and greater resistance to cellular stress.
We discovered that Stip1 serves as a central regulator of proteostasis, the cell’s system for maintaining protein health and stability, which is crucial for pluripotency and development. Essentially, Stip1 acts as a guardian of stem cells, governing their ability to divide, survive, and maintain genomic integrity.
On a mechanistic level, we demonstrated that Stip1 influences proteins involved in cell cycle progression and DNA damage response, providing a molecular basis for its essential role.
Our work establishes Stip1 as a pivotal regulator of embryonic stem cells, drawing attention to a role that is still little explored, but extremely relevant, of this protein in embryonic development. This journey, from studying a simple protein interaction to uncovering a factor vital for life, highlights how following scientific curiosity can lead to fundamental discoveries with broad implications, including for regenerative medicine where stem cell resilience is key.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in