Toward waterproof magnesium metal anodes by uncovering water-induced passivation and drawing water-tolerant interphases
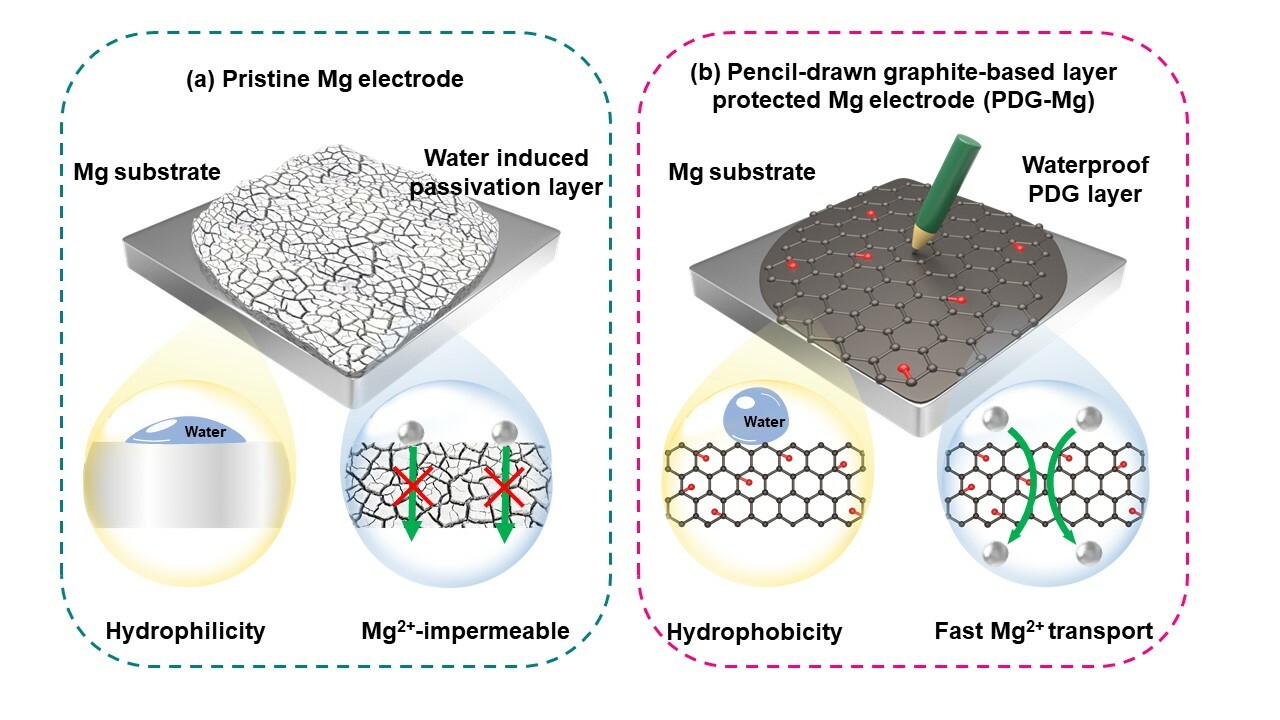
Research background: Rechargeable magnesium (Mg) metal batteries are a promising candidate for “post-Li-ion batteries” due to their high capacity, high abundance, and most importantly, highly reversible and dendrite-free Mg metal anode. However, Mg metal anode suffers from severe surface passivation in conventional electrolytes, which is closely related to side reactions from water contaminants. Although massive efforts have been devoted to mitigate the adverse impact of H2O impurities on the electrolyte-Mg metal anode interfacial passivation, there is still lack of knowledge of the chemical composition and microstructure of the passivation layer, which is essential to fundamentally analyze the mechanism of Mg anode failure.
Our research: In this work, we uncover the water-induced passivation mechanism and confirm that the passivation of Mg anode is accompanied by the hydrogen evolution reactions, and more importantly, the released gases can further react with Mg metal to form MgH2. Notably, the passivation by-product MgH2 has not been reported before on Mg metal anodes. The spatial distribution of MgH2 and its adverse impact on Mg2+ transport through the passivation layer is revealed for the first time by using time-of-flight secondary ion mass spectrometry (ToF-SIMS) and theoretical calculations/simulations. As a result, the passivation layer consisting of MgH2, along with widely explored MgO and Mg(OH)2, was found to induce intense concentration polarization at the electrode/electrolyte interface, ultimately causing high overpotential (> 2 V) and rapid failure (< 1 cycle) of water-attacked Mg metal.
With this improved mechanistic understanding, we also demonstrated the first waterproof Mg anode by drawing a graphite-based anti-passivation interphase on the Mg metal surface with a pencil. With its inherent hydrophobicity, high magnesiophilicity, and rapid Mg2+ diffusivity, the pencil-drawn graphite (PDG) interphase not only efficiently avoids water invasion, but also significantly increases Mg plating/stripping reversibility by homogenizing the distribution of ion flux and electric field at the electrolyte-electrode interface. Notably the PDG-Mg metal anode, even after water treatment, is still capable of working steadily for over 900 h in a symmetric cell and over 500 cycles in the full cell using a Chevrel phase Mo6S8 cathode. The practicability of the waterproof PDG-Mg anode is also extended to a simple aqueous electrolyte.
These insights gained from the identification of MgH2 in the passivation layer, the new mechanistic understanding of Mg passivation by H2O, as well as the construction of anti-passivation interphase can inspire the design of next-generation high-energy metal anodes with high water stability toward practical application.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in