Translational and Clinical Comparison of Whole Genome and Transcriptome to Panel Sequencing in Precision Oncology
Published in Cancer, Protocols & Methods, and Biomedical Research
Our study stemmed from the Molecularly Aided Stratification for Tumor Eradication (DKFZ/NCT/DKTK MASTER) program, a multicenter clinical sequencing initiative in Germany, focusing on comprehensive genomic profiling for patients with both rare as well as advanced cancers under the age of 51 (1-3). The MASTER program employs whole-exome/genome (WES/WGS) and transcriptome sequencing (TS) to uncover actionable molecular biomarkers, discussing molecularly informed therapy approaches weekly in a multidisciplinary molecular tumorboard (MTB). While comprehensive sequencing offers unparalleled insights, targeted gene panels, which are faster and more cost-effective, remain the standard in clinical settings. This prompted us to compare these methods head-to-head.
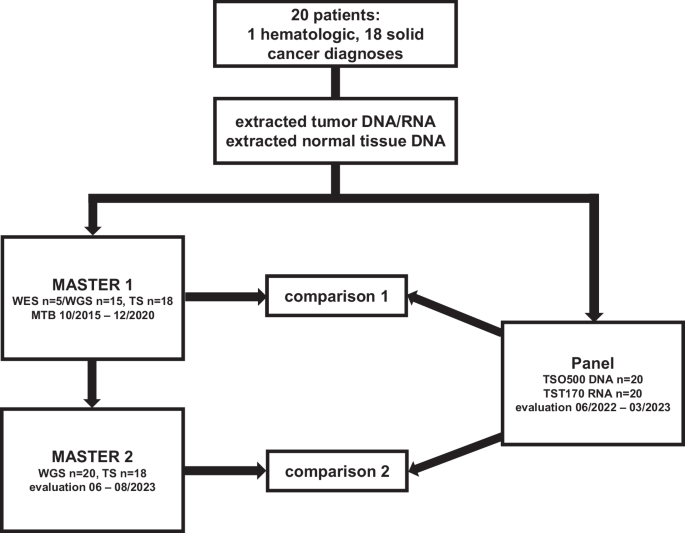
We selected 20 patients with 19 different advanced or metastasized tumor entities previously sequenced by WES/WGS ± TS in the MASTER program from 2015 to 2020 (MASTER 1) and resequenced their tumor DNA and RNA, alongside germline DNA, using the TSO500 DNA/TST170 RNA panel assays, which cover 523 genes involved in tumorigenesis at the DNA level for single nucleotide variations (SNVs), small insertions or deletions (indels), and 59 genes for copy number variation (CNV) detection, as well as 55 genes for the detection of fusions and splice variants at the RNA level (4). In an effort to homogenize and update WES/WGS ± TS sequencing data, as well as to mitigate temporal differences from the panel analysis, reanalysis of the original sequencing data was conducted using the current MASTER program bioinformatics pipeline in June 2023 (MASTER 2). Therapy recommendations (TRs) were independently issued for both the panel and the MASTER 2 analysis based on the current state of research at the respective time of assessment (evaluation panel: 06/2022-03/2023, evaluation MASTER 2: 06-08/2023; Fig. 1). TRs and their underlying biomarkers (BMs) derived from the panel were compared to TRs and underlying BMs from MASTER 1 (comparison 1) and MASTER 2 (comparison 2).
Key Findings
Our results revealed that approximately half of the therapy recommendations from the two sequencing approaches were identical (47.1% of the TRs of MASTER 1 and 62.8% of the TRs of the panel in comparison 1 as well as 45.9% of TRs of MASTER 2 and 54.9% of the panel-derived TRs in comparison 2), underscoring the broad utility of panel sequencing (Figure 1). However, a third of the WES/WGS ± TS-based recommendations (26.5% and 36.1% in MASTER 1 and MASTER 2, respectively) relied on BMs that were absent in the panel, with a significant portion based entirely on RNA expression data (72.2% in MASTER 1; 59.1% in MASTER 2), followed by alterations in genomic regions outside the panel’s capture area (22.2% and 36.4% respectively), and composite BMs such as DNA mutational signature and score calculations for homologous recombination deficiency.
Two therapy implementations of multi targeted tyrosine kinase inhibition in two patients were issued based on RNA expression data and a fusion with genes outside the panel’s target capture or RNA expression data alone, and therefore could not be identified by the panel.
These findings emphasize the broader application of WES/WGS ± TS in identifying composite molecular features and RNA-based alterations, which are often crucial for molecularly informed therapies in advanced cancers.
While the practical advantages of panel sequencing, such as its generally lower cost and compatibility with archived tissue samples, cannot be ignored, it is important to note its limitations, which become apparent in our study. Panel sequencing offers efficient and targeted molecular insights within predefined parameters, whereas whole-genome and transcriptome sequencing provides a comprehensive, multidimensional approach with an almost infinite range of biomarkers in terms of numbers and complexity. This broader capability, combined with the potential for multiomics integration, highlights its promise for more personalized oncology strategies and the future development of advanced therapeutic approaches.
Challenges and Future Directions
A critical insight from this study was the trade-off between the depth of molecular profiling and clinical feasibility. While WGS and TS offer unparalleled detail, their adoption in routine clinical practice faces barriers, including cost, processing time, and tissue requirements. Meanwhile, panel sequencing's limited biomarker capture restricts its applicability in more complex cases.
Our findings underline the necessity for randomized, controlled studies with larger patient cohorts to conclusively determine the socioeconomic impact of these sequencing strategies. Additionally, continued advancements in sequencing technologies and data analysis pipelines will likely further bridge this gap.
Reflections
Writing this paper was a collaborative journey, bridging molecular oncology, bioinformatics, and clinical practice. It was both challenging and rewarding to critically evaluate these sequencing methods and consider their implications for real-world cancer care.
We hope our study sparks further dialogue on the role of comprehensive genomic profiling in oncology and encourages clinicians and researchers to adopt a tailored approach based on patient needs and resource availability.
Literature
- Horak, P. et al. Comprehensive Genomic and Transcriptomic Analysis for Guiding Therapeutic Decisions in Patients with Rare Cancers. Cancer Discov. 11, 2780–2795 (2021).
- Mock, A. et al. NCT/DKFZ MASTER handbook of interpreting whole-genome, transcriptome, and methylome data for precision oncology. NPJ Precis. Oncol. 7, 109 (2023).
- Jahn, A. et al. Comprehensive cancer predisposition testing within the prospective MASTER trial identifies hereditary cancer patients and supports treatment decisions for rare cancers. Oncol. 33, 1186–1199 (2022).
- Zhao, C. et al. TruSight Oncology 500: Enabling Comprehensive Genomic Profiling and Biomarker Reporting with Targeted Sequencing. http://biorxiv.org/lookup/doi/10.1101/2020.10.21.349100 (2020) doi:10.1101/2020.10.21.349100.
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in