Unveiling the Mechanisms Behind Cancer Aggressiveness: How Epichaperomes Drive Cellular Plasticity
In our latest study published in Nature Communications, we explore a fundamental mechanism that governs the aggressive behavior of cancer cells. Specifically, we investigated a class of protein assemblies called epichaperomes, which are tightly bound networks of chaperones that enable cancer cells to reorganize their protein networks under stress [1,2]. Our findings shed light on how these structures contribute to cancer cells’ ability to adapt, survive, and proliferate by reactivating processes typically associated with developmental stages, such as pluripotency.
Understanding Epichaperomes: A Critical Player in Cancer Plasticity
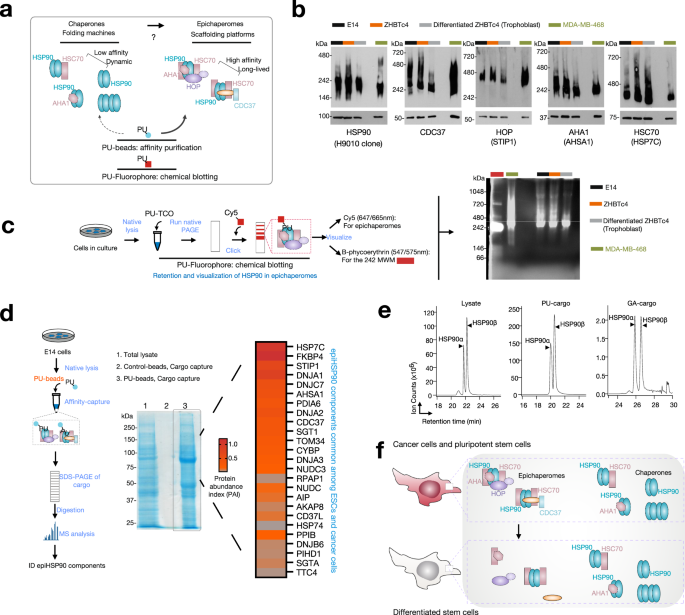
Epichaperomes are scaffolding platforms that form under stress conditions and reorganize protein interactions. While chaperones typically function to help fold proteins and maintain cellular proteostasis, epichaperomes go beyond this role by restructuring protein networks, allowing cells to rapidly adapt to changing environments. This study reveals that the assembly of epichaperomes is driven by specific post-translational modifications (PTMs) of the chaperone protein HSP90, particularly phosphorylation at two key residues (Ser226 and Ser255) [3].
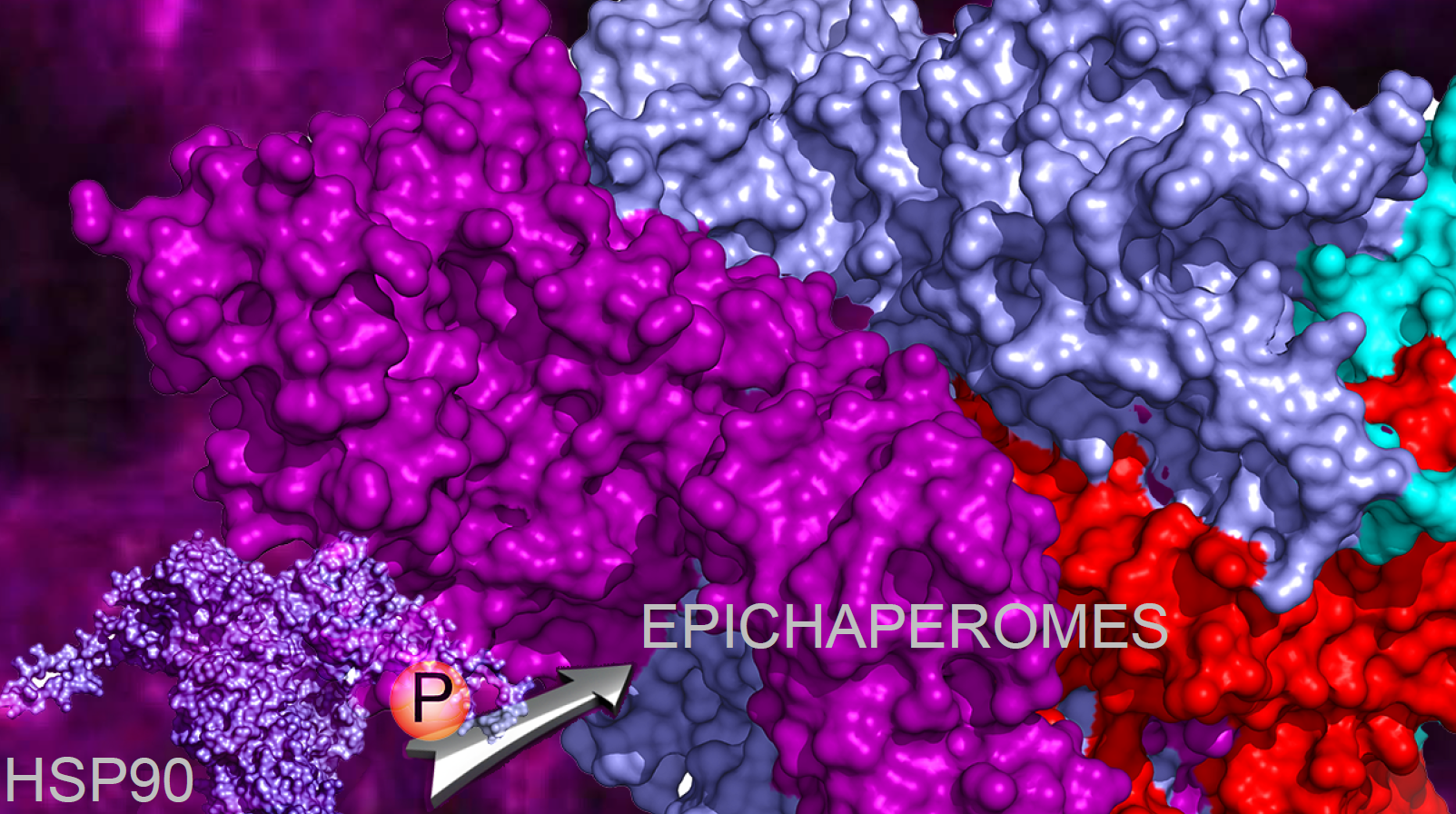
From Stem Cells to Cancer: A Shared Mechanism of Adaptation
What makes our findings particularly significant is the shared function of epichaperomes between pluripotent stem cells and cancer cells. In both cases, the ability of these cells to proliferate and adapt relies on epichaperomes to regulate protein networks. We found that phosphorylation of HSP90 enhances its interactions with other chaperones, co-chaperones, and client proteins, creating a microenvironment conducive to the formation of epichaperomes.
In cancer cells, this mechanism allows the reactivation of processes associated with developmental stages, contributing to cellular plasticity and the maintenance of a stem-like state. This plasticity enables cancer cells to transition between different phenotypic states, resist treatment, and become more aggressive.
The Role of CK2 in Epichaperome Formation
A key aspect of our study is the identification of CK2 as a kinase responsible for the phosphorylation of HSP90. CK2 is frequently overexpressed in cancers and is known to be involved in various disease processes. By promoting the formation of epichaperomes, CK2 plays a central role in enabling the plasticity and adaptability of cancer cells. This insight opens up new possibilities for targeting CK2-driven epichaperome formation as a therapeutic strategy.
Implications for Precision Medicine
Our findings have important implications for the development of novel therapies. By selectively targeting the epichaperomes in cancer cells—without affecting the normal functions of HSP90 in healthy cells—it may be possible to disrupt cancer cells’ ability to survive and proliferate under stress. This precision-targeted approach has the potential to enhance the efficacy of existing cancer treatments while minimizing side effects.
Moving Forward
This research represents an exciting step toward understanding how cancer cells exploit mechanisms of adaptability, such as epichaperomes, to sustain their aggressive behavior. We hope that these insights will lead to new therapeutic strategies that target the plasticity and adaptability of cancer cells, ultimately improving outcomes for patients with aggressive cancers.
We’re excited to continue exploring the role of epichaperomes in other diseases and to further unravel how these structures could be leveraged for therapeutic interventions.
References
[1] Chiosis G, Digwal CS, Trepel JB, Neckers L. Structural and functional complexity of HSP90 in cellular homeostasis and disease. Nat Rev Mol Cell Biol. 2023 Nov;24(11):797-815. doi: 10.1038/s41580-023-00640-9. Epub 2023 Jul 31. PMID: 37524848; PMCID: PMC10592246.
[2] Rodina A, Wang T, Yan P, Gomes ED, Dunphy MP, Pillarsetty N, Koren J, Gerecitano JF, Taldone T, Zong H, Caldas-Lopes E, Alpaugh M, Corben A, Riolo M, Beattie B, Pressl C, Peter RI, Xu C, Trondl R, Patel HJ, Shimizu F, Bolaender A, Yang C, Panchal P, Farooq MF, Kishinevsky S, Modi S, Lin O, Chu F, Patil S, Erdjument-Bromage H, Zanzonico P, Hudis C, Studer L, Roboz GJ, Cesarman E, Cerchietti L, Levine R, Melnick A, Larson SM, Lewis JS, Guzman ML, Chiosis G. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016 Oct 20;538(7625):397-401. doi: 10.1038/nature19807. Epub 2016 Oct 5. PMID: 27706135; PMCID: PMC5283383.
[3] Roychowdhury, T., McNutt, S.W., Pasala, C. et al. Phosphorylation-driven epichaperome assembly is a regulator of cellular adaptability and proliferation. Nat Commun 15, 8912 (2024). https://doi.org/10.1038/s41467-024-53178-5
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in