Unveiling the Mysteries: Mechanism of Allosteric Inhibition of Human p97/VCP ATPase and Its Disease Mutant by Triazole Inhibitors
Published in Cell & Molecular Biology and Pharmacy & Pharmacology
Introduction
Understanding the intricacies of protein mechanisms is akin to not only unlocking the secrets of life itself but also providing design concepts for drugs in treating critical diseases. Our recent paper, "Mechanism of Allosteric Inhibition of Human p97/VCP ATPase and Its Disease Mutant by Triazole Inhibitors," published in Communications Chemistry, delves into one such enigmatic mechanistic pathway. This study sheds light on the allosteric inhibition of the human p97/VCP ATPase, a key player in diverse cellular processes, by triazole inhibitors. The implications of our findings are far-reaching, with potential applications in therapeutic interventions for various diseases.
The Journey Begins: Inspiration and Background
The initial spark for this research derived from past scientific literature and extensive search for small molecules that inhibit p97 ATPase activity. The human p97 ATPase, also known as valosin-containing protein, plays a pivotal role in multiple cellular processes such as protein degradation, endosomal sorting, membrane fusion, and chromosome remodeling. Mutations in p97 are linked to several critical diseases, including a multisystem degenerative disorder known as inclusion body myopathy with early-onset Paget disease and frontotemporal dementia (IBMPFD) and familial amyotrophic lateral sclerosis (fALS). The pressing need to understand how to modulate the activity of p97, especially in its mutant forms, led us to explore potential inhibitors.
Diving Deep: The Mechanism of Allosteric Inhibition
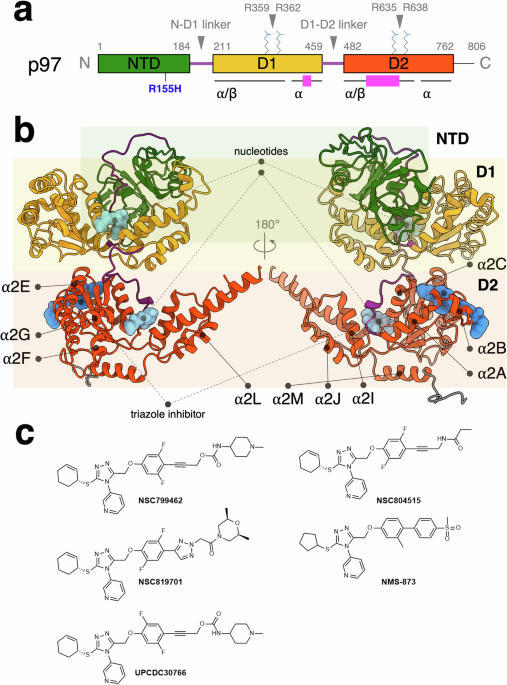
Our primary focus was allosteric inhibition, where a molecule binds to a protein at a site distinct from the active site, altering the protein's function. The active-site inhibitor of the earlier generation produces undesired mutational resistance, and the use of the allosteric inhibitor bypasses this resistance and maintains the reduction of the ATPase activity when used in parallel. We aimed to decipher how a set of newly synthesized triazole inhibitors modulate the activity of p97 and a handful of its disease mutants. To comprehensively investigate this allosteric inhibition, our approach was multifaceted, involving a combination of organic chemistry, biochemistry, structural biology, and cell biology.
Structural Insights: Cryo-EM
One of the most exciting aspects of our study was utilizing cryogenic electron microscopy (cryo-EM) to capture high-resolution structural details of wild-type and mutant forms of p97 in a complex with triazole inhibitors. These structures provided a detailed view of the binding sites and conformational changes induced by the inhibitors. Our study is the first study to compare the triazole inhibitor binding between the wild-type and mutant p97, which have differential ATPase activities between normal and cancer cells.
The resulting structures reveal the binding sites of the triazole inhibitors and their impact on the structural changes of the p97 ATPase, providing mechanistic insight into the inhibition of its ATPase activity. Furthermore, specific interactions between the inhibitors and amino acid residues at the allosteric site are identified, which are critical to influencing the intra- and inter-domain communications within the protein complex.
Biochemical Characterization: Activity Assays
To complement our structural studies, a series of biochemical assays were conducted to measure the ATPase activity of wild-type and mutant p97 in the presence of triazole inhibitors. These assays demonstrated a significant reduction in ATPase activity, confirming the inhibitory effect observed in our structural analyses.
The Impact: Therapeutic Potential and Beyond
The implications of our findings extend beyond basic biochemical knowledge. The ability to selectively inhibit p97 and its disease mutants opens new avenues for therapeutic intervention. Diseases such as fALS and IBMPFD, which are linked to dysfunctional p97, could potentially be treated by targeting the allosteric sites identified in our study. Moreover, our research provides a framework for developing allosteric inhibitors for other ATPases and molecular machines.
Reflecting on the Journey: Challenges and Triumphs
Every research journey is fraught with challenges, and ours was no exception. The obstacles and challenges in this study, such as handling the poor solubility of the lead compounds and processing heterogeneous structural data of the target protein complexes, were successfully overcome through the persistence and collaboration of our multidisciplinary team. The integration of approaches in chemistry, biochemistry, structural biology, cell biology, and computation was instrumental in unraveling the complex mechanism of allosteric inhibition.
Conclusion
The publication of our paper in Communications Chemistry marks a significant milestone in our quest to understand the allosteric inhibition of p97. Our findings not only elucidate a crucial biochemical mechanism but also pave the way for developing novel therapeutic strategies. This journey, driven by curiosity and collaboration, underscores the importance of multidisciplinary approaches in tackling complex biological problems. We look forward to seeing how our structural insights may aid in designing next-generation inhibitors with enhanced potency and selectivity.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in