When cancer cells get squeezed: discovering how physical forces shape metastasis
Published in Bioengineering & Biotechnology, Cancer, and Biomedical Research
Why we cared about this question
Most people know that cancer is dangerous because it spreads. What is less widely appreciated is how difficult that journey is for cancer cells. Once in the bloodstream, tumor cells are swept along at high speed, attacked by the immune system, and starved of nutrients. Most don’t survive.
And yet, a small fraction does; these rare survivors travel to new organs and seed metastases, which are responsible for nearly 90% of cancer deaths.
One mystery has puzzled researchers for decades: why do so few cells succeed, and what makes them different from the rest?
Looking at the problem differently
As engineers and biologists working together, we wondered if the answer might not just lie in genetics or biochemistry, but in something simpler: the physical experience of traveling through the body.
To reach distant tissues, circulating tumor cells must pass through capillaries , the body’s tiniest blood vessels, often narrower than the cell itself. Imagine trying to squeeze a balloon through a drinking straw: it bends, stretches, and sometimes bursts. We asked ourselves: what if this squeezing isn’t just a barrier, but a trigger for change?
A chip that mimics blood vessels
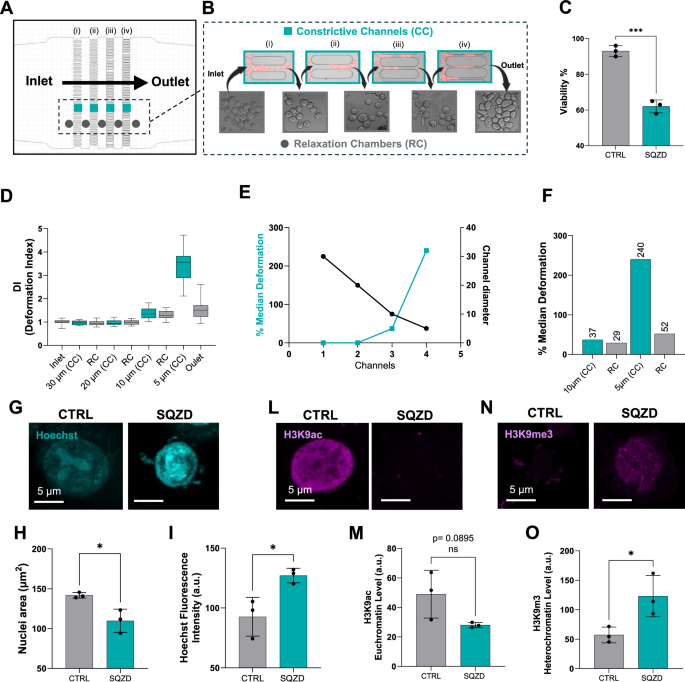
Studying this process inside the body is almost impossible. Cells are too small and too few to track in real time. So we turned to microfluidics, building a tiny chip with channels that gradually narrow down to the width of a human capillary.
With this model, we could actually watch cancer cells get squeezed under a microscope and then follow what happened to them afterwards.
The big surprise
We expected some cells would be damaged by the stress, and indeed many died. But what surprised us most was what happened to the survivors.
The cells that made it through the constrictions didn’t just carry on as before, they seemed reprogrammed. They switched states that help them survive hostile environments, interact with blood vessels, and seed new tumors. In other words, squeezing appeared to transform ordinary cancer cells into a more dangerous, stem cell–like state.
This was not a temporary effect. When we grew these “squeezed” cells in the lab, they formed bigger tumor-like spheres. When we tested them in animal models, they spread faster and seeded more metastases than control cells.
The hidden switch: a mechanosensor called PIEZO1
How do cells know they’ve been squeezed? The answer came from a fascinating protein called PIEZO1, a “mechanosensor.” It acts like a pressure valve, opening when the cell membrane is stretched and letting calcium ions rush inside.
That simple mechanical cue sets off a cascade of signals that reprograms the cell. We showed that blocking PIEZO1 stopped the transformation. Even more striking, artificially activating PIEZO1 with a drug pushed the cells into the same aggressive state — without any squeezing at all.
This revealed that PIEZO1 is a kind of master switch, translating the physical world of force and pressure into the biological language of cancer progression.
Why this matters
For decades, cancer research has focused on mutations and chemical signals. Our work adds a new piece to the puzzle: mechanical forces can also drive cancer evolution.
This changes how we think about metastasis. Capillaries aren’t just passive obstacles; they may actively “train” cancer cells to become better at colonizing new sites.
From a clinical perspective, this opens intriguing possibilities. If drugs could block PIEZO1, perhaps we could prevent circulating tumor cells from becoming more aggressive — cutting metastasis off at its earliest stages.
Looking forward
Our study answers one question but raises many more. Do other cancers respond the same way? Could PIEZO1 inhibitors really reduce metastatic spread in patients? And might physical forces in other organs, like compression in the brain or lungs, play a similar role?
We are only beginning to appreciate the role of mechanics in biology. Cancer cells, it turns out, are not just genetic or chemical entities; they are also physical objects, shaped by the forces around them.
Exploring this mechanical dimension may reveal vulnerabilities we’ve been missing and, we hope, new ways to stop metastasis before it takes hold.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in