Why not both? Combined use of light and redox enables adaptive switching of responsive hydrogels

The integration of molecular switches into materials has emerged as a powerful approach to generate stimuli-responsive systems with numerous potential applications in energy or information storage, actuators and soft-robotics.
To this end, light-driven switches are well-established responsive building blocks, as light constitutes a convenient, external and waste-free means of controlling materials with high spatio-temporal control.1 Conceivably, redox switches could fulfill a similar role and thereby provide alternative or complementary switching functions, characterized by large changes in charge and associated properties. However, this redox-switching approach is noticeably underdeveloped, which can, at least in part, be attributed to the lack of redox switches that can be reversibly switched over multiple cycles in the presence of water and oxygen.
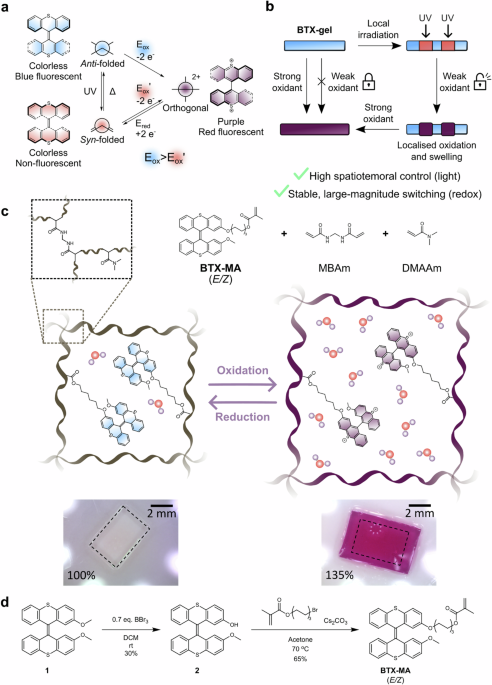
We realized that BTX, a simple overcrowded alkene, may be ideal in this context, as its two-electron redox process avoids the formation of reactive radical intermediates, enabling highly reversible switching between two chemically stable states that possess very different geometries, charge, color and fluorescence.2 Its integration into a hydrogel matrix was synthetically comparably straight-forward and was achieved by light-induced radical polymerization. After some optimization of the unresponsive, diluent co-monomer as well as the ratio of crosslinker and BTX-switch, a composition was found in which the resulting hydrogel was stiff enough to be easily manually manipulated without breaking, while being soft enough to allow for expansion by swelling. Indeed, this initial goal of redox-driven gel expansion was quickly achieved; upon exposure of the material to the strong oxidant ceric ammonium nitrate (CAN) in water, the gel immediately changes color from transparent to purple/red due to the oxidation of the BTX switches throughout the whole material (Figure 1). In addition, its fluorescence also changes from bright blue to weak red, and most notably, the gel expands isotropically by up to 146% in terms of volume.
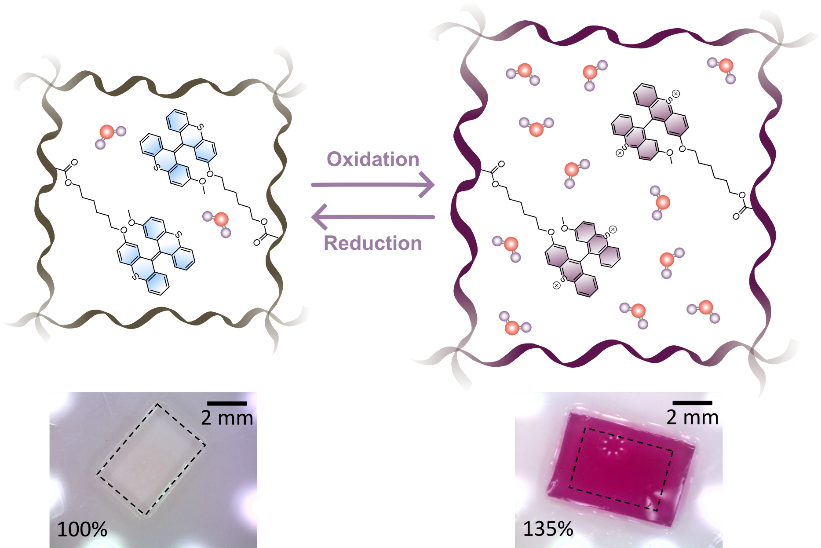
The large change in size can be attributed to the significant increase of charge of the gel and the resultant ingress of water. This was then exploited to construct numerous different actuators, such as helical springs or tubes, whose morphology changes significantly and reversibly; reduction with ascorbate restored the initial size and color of the gel pieces (see video).
At the same time we explored the BTX switching scaffold in various other projects and applications3-6 and noticed that its switching properties are even richer. Specifically, in addition to the above-described dicationic and neutral anti-folded states a third switching state, the neutral syn-folded state can also be generated, either by irradiation of the lowest energy anti-folded state or by reduction of the dication (Figure 2A). Due to its metastability, this syn-folded state quickly relaxes back to the anti-folded state in the absence of light; reduction of the oxidized state thus results in a pre-defined, autonomous three-state switching cascade (dication → syn → anti).3,4 We then wondered if this sequence can also be driven in the opposite direction and if this may be useful. Indeed, light-induced anti → syn switching, followed by syn → dication oxidation is a possible “reverse” pathway, as already shown nearly two decades ago.2
But how can this be turned from an interesting fundamental property into a useful function, especially in a material?
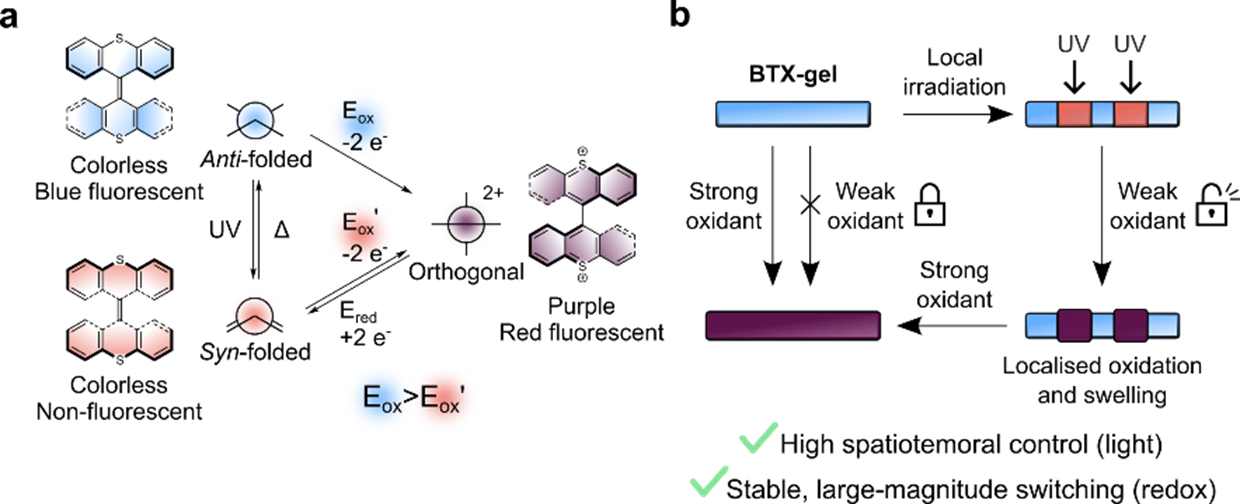
Figure 2. A) Interconversion between the three switching states of BTX. B) Light-gated redox switching of BTX-gel.
UV-light irradiation of the BTX gel also induces efficient conversion to the syn-folded state, however this only turns off the fluorescence of the gel and does not, in contrast to redox-switching, induce any color or morphological changes. However, this light-switching induces one very important property change: the potential for the two-electron oxidation of the syn-folded state is significantly lower than that of the anti-folded state.
We surmised that this unique interplay of light-switching and redox properties can be leveraged to overcome one of the inherent limitations of redox switching, the lack of spatial control over redox. By chemical redox, as used herein, it is very difficult to selectively address only parts of a material, and electrochemical redox using electrodes suffers from similar problems and is generally limited to 2D interfaces. However, light does not suffer from this problem and can be locally applied, e.g. by photomasking. Hence, upon local light-switching of the gel, the local redox properties are changed, such that selective oxidation is possible (Figure 2B). Having realized that this unique light-gated redox patterning approach should be viable, the most obvious hurdle to experimentally demonstrate this principle was finding a suitable chemical oxidant that on one hand is sufficiently strong to oxidize the irradiated, light-switched area, i.e. the syn-folded state, but not strong enough to oxidize the rest of the material, i.e. the anti-folded state. We anticipated that this might prove a significant challenge, but to our delight, the first oxidant we tested, iron(iii)perchlorate immediately fulfilled these criteria! When exposing the native gel to this oxidant nothing happens at all, however upon (localized) irradiation with UV light, immediate and long-term stable oxidation to the dication with concomitant local coloration and swelling was observed, corresponding to the above-mentioned ”reverse” switching cascade. This adaptivity7 enabled us to combine the advantage of both light switching (high spatio-temporal precision) with those of redox switching (large scale and long-term stable changes of the material) thereby generating versatile hydrogel materials that can be reversibly programmed to generate various complex patterns, motions and even surface textures (Figure 3).

Figure 3. Patterning of hydrogels via light-gated redox switching.
Check out the paper for more details about our light-gated redox switching approach in responsive hydrogels!
Written by Robert Hein with edits from Roza Weber.
- Goulet-Hanssens, A., Eisenreich, F. & Hecht, S. Enlightening materials with photoswitches. Mater. 32, 1905966 (2020).
- Browne,W. R., Pollard, M. M., De Lange, B.,Meetsma, A. & Feringa, B. L. Reversible three-state switching of luminescence: A new twist to electro- and photochromic behavior. J. Am. Chem. Soc. 128, 12412–12413 (2006).
- Hein, R., Gisbert, Y. & Feringa, B. L. Multi-state redox and lightdriven switching of pseudorotaxanation and cation shuttling. Am. Chem. Soc. 147, 13649–13657 (2025).
- Hein, R., Sidler, E., Gisbert, Y. & Feringa, B. L. Chiral Induction and Memory via Supramolecular Deracemization. Chem. Int. Ed. 64, e202510584 (2025).
- Sidler, E., Hein, R., Doellerer, D. & Feringa, B. L. Redox-switchable aromaticity in a helically extended indeno[2,1- c]fluorene. Am. Chem. Soc. 146, 19168–19176 (2024).
- Hein, R., Stindt, C. N. & Feringa, B. L. Mix and match tuning of the conformational and multistate redox switching properties of an overcrowded alkene. Am. Chem. Soc. 146, 26275–26285 (2024).
- Kaspar, C., Ravoo, B.J., van der Wiel, W.G. et al. The rise of intelligent matter. Nature 594, 345–355 (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in