With Carbon Nitride Photocatalysis toward “Shiny” Molecules from Bifunctional Precursors
Published in Chemistry
Science is awesome! When we are persistent enough it rewards us with nice "gifts". There is an old scientific joke: "Theory is when you know everything, but nothing works. Practice is when everything works, but no one knows why. In our lab theory and practice combined – nothing works and no one knows why". Did it happen to you that you struggle on some topic for months, but no progress? I am sure it happened. It happened to all of us! Like gold prospectors we rinse tons of mud in a search of precious gems. Among the routine work sometimes unexpected, but fascinating discoveries do happen. And then fun starts. The task of scientist is to explain how and why does this or that phenomenon occur. This case study is about finding the biggest scientific gem in the history … of our lab.
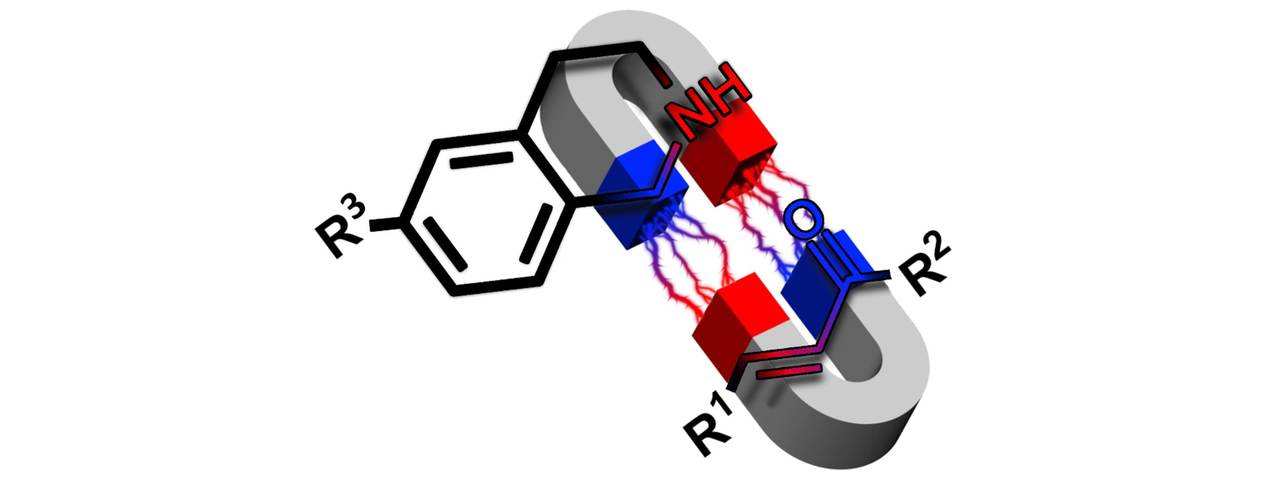
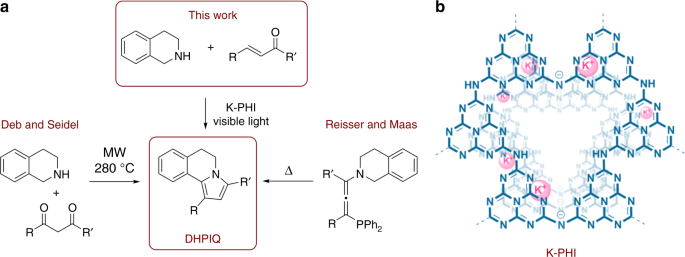
Our lab focuses on application of carbon nitrides in organic photocatalysis. In photocatalysis one of the most studied reactions is functionalization of N-aryltetrahydroisoquinolines at the benzylic position with nucleophiles – nitromethane, malone esters etc. The reaction proceeds via N-aryltetrahydroisoquinolinium cation generated by the photocatalyst (PC) and O2. The N-aryltetrahydroisoquinolinium cation then reacts with the nucleophiles in the Mannich reaction.
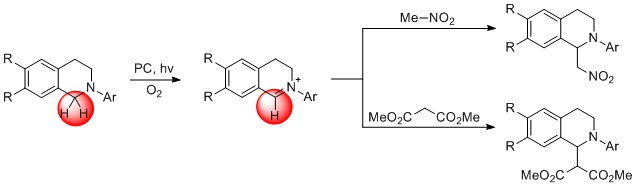
After a thorough literature survey we found that there are no reports of using bare tetrahydroisoquinolines (THIQs) in photo-Mannich reaction. In principle bare THIQ offers two reactive sites – NH-function and activated benzylic -CH2- group. Therefore, when combined with the appropriate bifunctional reagent, for example, chalcone, it could react in a concerted way and presumably give structurally interesting organic molecule.
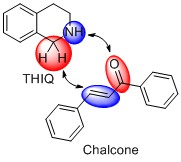
By mixing THIQ, chalcone, carbon nitride photocatalyst and blue photons we obtained a compound that according to IUPAC rules has a complex name – 1,3-diphenyl-5,6-dihydropyrrolo[2,1-a]isoquinolines. But we simply call it DHPIQ. We expanded the scope of the reaction to 15 DHPIQs. Was it that easy? No! Here is some statistics on this project. From the very first try to the acceptance of the article for publication it took 208 experiments, 740 NMR spectra, 296 mL of deuterated solvents, 240 h of NMR measurement time, 960 man-hours. Amazingly, but it was done mostly by 2 people – Bogdan Kurpil and Katharina Otte!
During the peer-review process, the referee raised a natural question: "What is so special about the DHPIQs?" Surely there are hundreds of different classes of organic compounds and millions of individual compounds. What is interesting about DHPIQs? This question prompted us to dig deeper into DHPIQs properties. We characterized DHPIQs by different physico-chemical methods and indeed found that certain DHPIQs have quantum yield of fluorescence up to 24%. Indeed, they shine in UV!
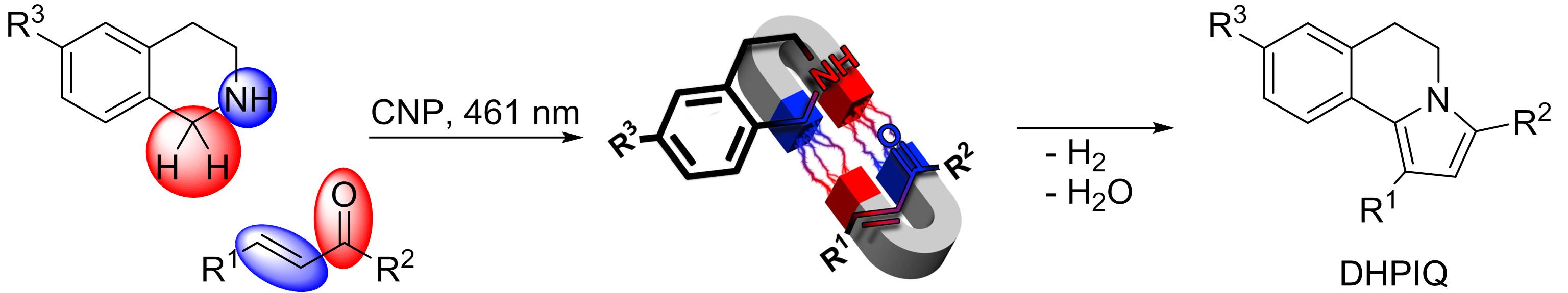
DHPIQ project would be uncompleted without addressing the question of the most demanding reviewer – our thirst for understanding how does this reaction work. This question was addressed combining multiple experimental and theoretical techniques in a very fruitful collaboration with talented researchers, Paolo Lamagni and Nina Lock from Aarhus University, Denmark and Artem Mishchenko from the V.I. Vernadsky Institute of General and Inorganic Chemistry, Kyiv, Ukraine. All experimental data and the results of the theoretical modelling suggest that carbon nitride photocatalyst (CNP) generates reactive intermediates from the THIQ and the chalcone. These intermediates are attracted by each other exactly as opposite poles of the magnets are attracted. Fusion of these intermediates followed by removal of H2 and H2O molecules yields DHPIQs.
Science tosses riddles and solving these riddles is the most exciting part of the research. The reason why we, scientists, wake up every morning even before sunrise to rush to our labs and offices to check new data, collected overnight from microquantities of scrupulously synthesized and purified samples, is to answer the question – "Why does it work?"
Are you interested to know the details why carbon nitride makes THIQs to react with the chalcones in a concerted fashion? Do you want to know more about optical and chemical properties of DHPIQs? Then check out our article “Carbon nitride photocatalyzes regioselective aminium radical addition to the carbonyl bond and yields N-fused pyrroles” published in the Nature Communications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in