A General Flame Aerosol Route to Kinetically Stabilized Metal-organic Frameworks
Explore the Research
Nature - Server Error
Sorry, the page you requested is unavailable.
Metal-organic frameworks (MOFs) have garnered attention across chemistry, physics, biology, and engineering. However, the vast majority of MOFs are currently synthesized through equilibrium liquid-phase reactions. Here, we present a general and versatile non-equilibrium flame aerosol synthesis of MOFs, in which rapid kinetics of MOF formation yields nano-crystalline MOFs, amorphous MOFs, and bi-metallic MOFs.
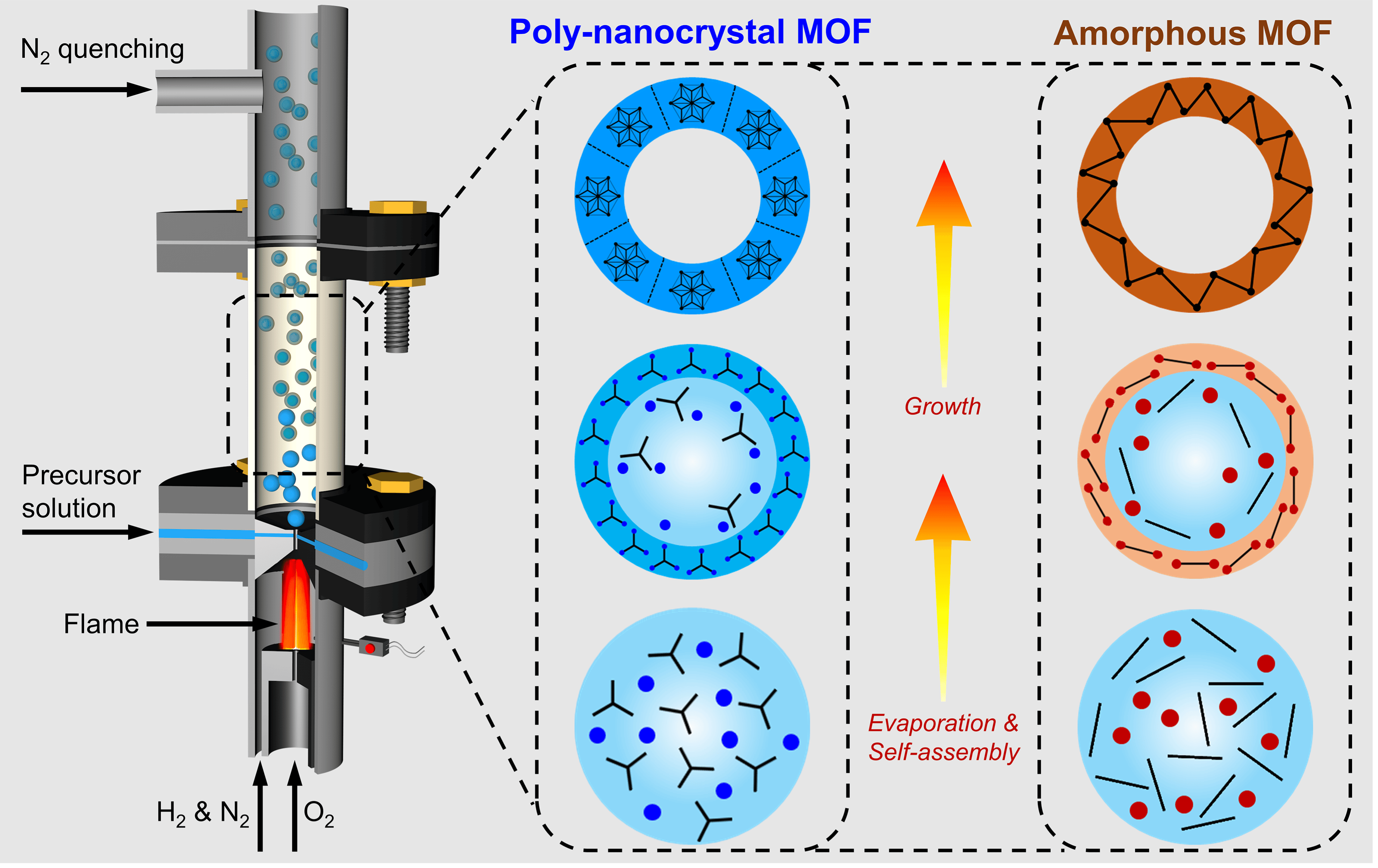
Figure 1. Schematic representation of nanocrystal and amorphous MOF formation in a non-equilibrium flame aerosol process.
What is the key breakthrough of the research published on Nature Communications by the team from University at Buffalo SUNY and Berkeley Lab?
A: The key breakthrough is a universal non-equilibrium flame aerosol synthesis to create MOFs. This is the first time that MOFs have been synthesized using flame aerosol (flame spray pyrolysis) technology.
By a rapid droplet-to-particle conversion, metal ions and organic ligands can rapidly assemble into spherical MOFs, and sometimes form hollow spherical structures. This method demonstrates exceptional versatility, enabling the synthesis of a wide range of nano-crystalline and amorphous MOFs. The produced MOFs also show exploitable features such as hollow nanoshell morphology, high grain boundary density, and short-range order structure. This approach opens up new opportunities for rational design of new MOF materials for various MOF-related applications.
What makes the flame aerosol synthesis method innovative compared to traditional MOFs synthesis methods?
A: Traditional MOF materials are typically synthesized via liquid-phase reactions under equilibrium conditions, producing highly crystalline single crystals. In contrast, the flame aerosol method developed in this study enables the formation of MOFs under rapid, non-equilibrium conditions, resulting in low-crystallinity materials, including nano-crystalline and amorphous MOFs. Although these MOFs have lower porosity than conventional highly crystalline MOFs, their unique properties—such as small grain size, short-range ordered structures, and high thermal stability—open up new applications distinct from those of traditional MOF materials.
More importantly, the far-from-equilibrium flame aerosol method circumvents thermodynamic barriers, enabling the mixing of any two metal elements into a single-phase MOF crystal, resulting in the formation of bimetallic MOFs or MOF-based single-atom catalysts. These properties are highly advantageous for applications in catalysis, sensing, and energy storage, among others.
What is the exsolution phenomenon discovered in this study?
A: As mentioned above, this method enables the integration of any two metal elements into a single-phase MOF structure. This allows for the incorporation of easily reducible active metals, such as Pt or Pd, into an inert MOF matrix, such as Zr-based MOFs. Subsequently, using a reducing agent like NaBH4 in solution, Pt or Pd can be selectively exsolved from the Zr MOFs, forming highly dispersed nanoparticles on the MOF surface. These supported nanostructures exhibit strong catalytic activity; for example, the resulting Pt/Zr UiO-66-NH2 acts as an efficient CO oxidation catalyst, achieving 100% CO conversion at a low temperature of 130°C.
What are the implications of this research for industrial applications?
A: The scalability, low cost, single and continuous step of the flame aerosol process make it an ideal candidate for commercial production, in contrast to many liquid-phase synthesis methods that typically require several time-consuming batch operations. Another advantage of this non-equilibrium reaction is that the material's performance does not significantly degrade with scale-up. Currently, Praxair (now Linde) has scaled thermal nozzles to gas flows of at least 2,000 times those used in our laboratory-scale reactor. In practical production, higher gas flow rates, elevated precursor flow rates, and increased precursor concentrations can be used to scale up MOF yield without sacrificing material quality. Meanwhile, the O2 and N2 mixture could be replaced by air for industrial production, further reducing costs.
Who led the research?
A: The research was led by Dr. Shuo Liu as the first author, under the joint supervision of Dr. Chaochao Dun, Dr. Jeffrey J. Urban, and Dr. Mark T. Swihart. It represents a collaboration between the University at Buffalo, SUNY and the Lawrence Berkeley National Laboratory. This joint effort between University at Buffalo and LBNL signifies a significant advance in the rational design of materials.
For more details, please check out our paper on Nature Communications "A General Flame Aerosol Route to Kinetically Stabilized Metal-organic Frameworks"
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in