A new chemogenetic system using Ionotropic Receptors from the fruit fly Drosophila melanogaster
Published in Neuroscience
Animal nervous systems create thoughts and evoke behaviors through a network of neural circuits consisting of thousands to billions of cells. In neuroscience, there has long been a need for methods to perturb specific neuronal populations or circuits in this network to elucidate their functions. Many years ago, we developed immunotoxin-mediated cell targeting for conditional ablation of the specific cell types, which depends on the cytotoxic activity of recombinant immunotoxin that selectively targets cells expressing the human interleukin-2 receptor alpha-subunit (1). To date, this approach has been applied to various types of cells and circuits in the central nervous systems of rodents and non-human primates, significantly advancing our understanding of their behavioral functions (2, 3).
To clarify a causal relationship between the activity of the nervous system and behavior, both loss- and gain-of-function studies are needed. More recently, we started working to develop a unique technology for gain-of-function approaches: a high efficiency, excitatory chemogenetic tool that uses Ionotropic Receptors (IRs) from Drosophila melanogaster, ligand-gated ion channels that normally function in environmental chemosensation. IRs have evolved from ionotropic glutamate receptors (iGluRs), best known for their roles in synaptic transmission, but encompass large, highly divergent families in most insects (4). IRs form heteromeric complexes of a tuning receptor - which defines ligand specificity - and a co-receptor (5, 6). Within the Drosophila IR family, the complex of IR84a (tuning receptor) and IR8a (co-receptor) form a non-specific cation channel that responds to phenylacetic acid and activates Drosophila sensory neurons in a ligand-dependent manner (7). In earlier work, we generated transgenic mice expressing IR84a/IR8a in the catecholaminergic neurons, notably in the locus coeruleus (LC) in the pons, and demonstrated that these neurons exhibit novel excitatory responses to phenylacetic acid administered in the vicinity of the LC using electrophysiological, biochemical, and behavioral methods (8). To our knowledge, this was the first excitatory chemogenetic system using IRs, and we named it “IRNA” (IR-mediated neuronal activation, see Figure). Using INRA, we went on to discover that the activity of the noradrenergic neurons in the LC controls the speed of recall for past aversive experiences via a projection to the amygdala. This finding suggests that the noradrenergic projection is one of the most promising therapeutic targets in psychiatric disorders that impair the recall process of emotional memory, such as post-traumatic stress disorder.
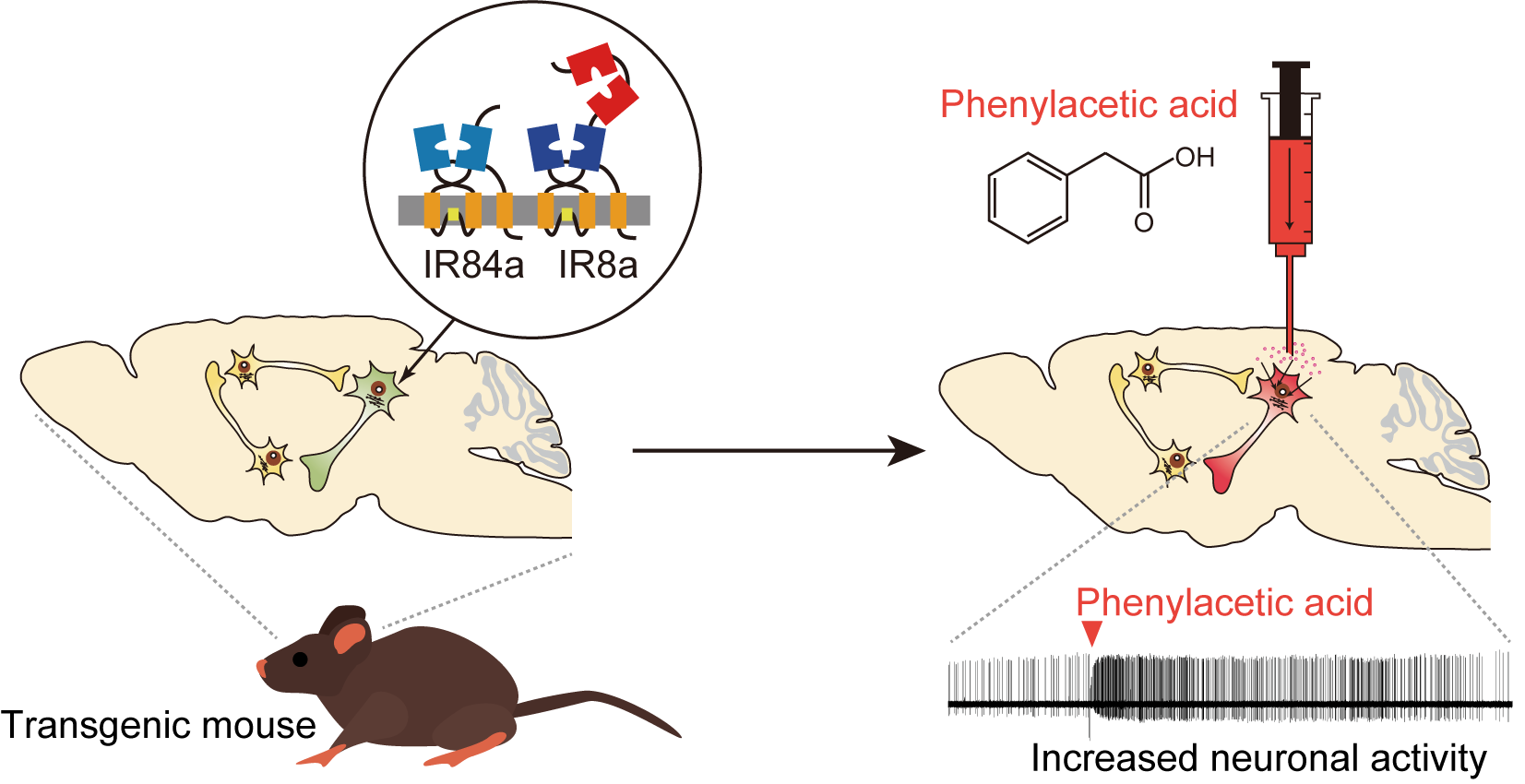
Figure: A schematic representation of the IRNA system. IR84a and IR8a expression is induced in
the target cell populations to confer excitatory responses to the artificially-applied cognate ligand
phenylacetic acid.
A substantial challenge of this initial system was the delivery of phenylacetic acid across the blood-brain barrier, requiring surgical treatment of animals and intracranial ligand infusion. To overcome this highly invasive administration with the IRNA system, in our new paper, we worked to develop a systemic pro-drug strategy to activate the target neurons by methyl esterification of phenylacetic acid, whose increased lipophilicity facilitated its translocation across the blood-brain barrier by passive diffusion, before being processed to the active acid form by endogenous esterase activity in the brain. When phenylacetic acid methyl ester was administered into the tail vein of the transgenic mice, we observed a noradrenergic activation in the brain manifested as increased spikes of the LC neurons and escalation of the transmitter release in the terminal region, indicating that our strategy does indeed work in vivo. In this paper, we further demonstrated, using a viral vector system, the induction of cell type-specific IR84a/IR8a expression in the striatal neurons of rats, which conferred sensitivity to peripheral administration of phenylacetic acid methyl ester. These findings indicate that the systemic pro-drug strategy can be extended beyond the LC neurons of mice to other neuronal types in other mammalian species.
Although there are many reports of chemogenetic tools based on other classes of ligand-gated ion channel, notably the Cys-loop receptors, excitatory systems to activate target cells in vivo are limited (9). P2X-based chemogenetics reported in invertebrates (10) necessitate a knockout of the endogenous receptor in mammalian applications. Our studies indicate that the IR family might help circumvent such a limitation of chemogenetic tools, not least because of the enormous functional diversity of these proteins, which have evolved to detect numerous external chemical signals (4, 5, 6). By modifying the composition of IR complexes, we can potentially create IRNA tools that respond to different ligands, with different pharmacokinetic properties, enabling precision in the desired artificial control of mammalian neuronal activity patterns.
References
- Kobayashi, K. et al. Immunotoxin-mediated conditional disruption of specific neurons in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 92, 1132-1136, doi; 10.1073/pnas.92.4.1132 (1995).
- Kato, S. et al. Action selection and flexible switching controlled by the intralaminar thalamic neurons. Cell Rep. 22, 2370-2382, doi: 10.1016/j.celrep.2018.02.016 (2018).
- Kinoshita, M. et al. Genetic dissection of the circuit for hand dexterity in primates. Nature 487, 235-238, doi: 10.1038/nature11206 (2012).
- Benton, R., Vannice, K. S., Gomez-Diaz, C. & Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149-162, doi: 10.1016/j.cell.2008.12.001 (2009).
- van Giesen, L. & Garrity, P. A. More than meets the IR: the expanding roles of variant Ionotropic Glutamate Receptors in sensing odor, taste, temperature and moisture. F1000Res. 6, 1753, doi: 10.12688/f1000research.12013.1 (2017).
- Ni, L. The structure and function of ionotropic receptors in Drosophila. Front. Mol. Neurosci. 13, 638839, doi: 10.3389/fnmol.2020.638839 (2020).
- Grosjean, Y. et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236-240, doi: 10.1038/nature10428 (2011).
- Fukabori, R. et al. Enhanced retrieval of taste associative memory by chemogenetic activation of locus coeruleus norepinephrine neurons. J. Neurosci. 40, 8367-8385, doi: 10.1523/jneurosci.1720-20.2020 (2020).
- Magnus, C. J. et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 364, doi: 10.1126/science.aav5282 (2019).
- Zemelman, B. V., Nesnas, N., Lee, G. A. & Miesenbock, G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc. Natl. Acad. Sci. U. S. A. 100, 1352-1357, doi: 10.1073/pnas.242738899 (2003).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in