Anti-Markovnikov hydroallylation reaction of alkenes via scandium-catalyzed allylic C‒H activation
Published in Chemistry, Earth & Environment, and Materials
The Origin of the Idea
Organo rare-earth catalysis have been emerging as a unique platform for various chemical transformations including the regio- and stereoselective C−H functionalization of heteroatom-containing compounds with alkenes and alkynes. In many of these transformations, the strong heteroatom-affinity of rare-earth metal ions and high reactivity of RE‒C bond towards unsaturated carbon-carbon bonds were essential to achieve reactivity and selectivity. In this context, auxiliary ligand is the core and key of rare-earth metal organic complex catalysis, which significantly affects the reactivity and selectivity of catalytic reactions. At present, cyclopentadiene (Cp)-based ligands occupied a dominated position.1 Except Cp and its derivatives, the diamine ligand by Mashima,2 the b-diimide ligand by Xu,3 and the phosphonamine ligand by Chen4 displayed good activity towards the hydroalkylation of pyridine derivatives by coordinating with rare-earth metals. Tamm, Inoue, Eisen, and others found that imidazolin-2-imine was a versatile class of ligands, which can coordinate with main group metals, transition metals and rare-earth metals to form the well-defined metal complexes.5 The theoretical calculations and experimental studies implied that imidazolin-2-iminato (NHI) anions can be served as Cp-like ligands. As shown in Figure 1, the two mesomeric structures of the imidazolin-2-iminato groups indicated that they can serve as 2σ,4π-type N-donor ligands. Thanks to their strong electron donation and steric tunability, the related imidazolin-2-iminato rare-earth alkyl complexes exhibit high activity toward several reactions, including hydroamination, hydrosilylation, nucleophilic addition, and polymerization. Nevertheless, it remains unclear whether imidazolin-2-iminato rare-earth alkyl complexes could be used in C–H activation. Motivated by the distinct selectivity and functional group tolerance frequently shown in rare-earth mediated C–H functionalization and elegant works from Tamm's group, we envisaged that the judicious choice of rare-earth ions and basic ligands, as well as modification of imidazolin-2-iminato supporting ligands, may have the potential to achieve C–H alkylation with olefins. An array of imidazolin-2-iminato rare-earth alkyl complexes were synthesized and structurally characterized by X-ray diffraction analysis. Recently, the cationic imidazolin-2-iminato scandium(III) alkyl complex was identified as an efficient catalyst for highly regioselective C–H alkylation of pyridine, anisole and their derivatives.6
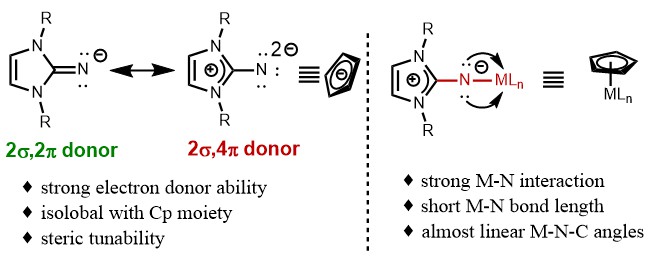
Figure 1. The structural features of N-heterocyclic iminato ligands.
A New Strategy: RE...π Interaction Promote Catalytic C−H Functionalization
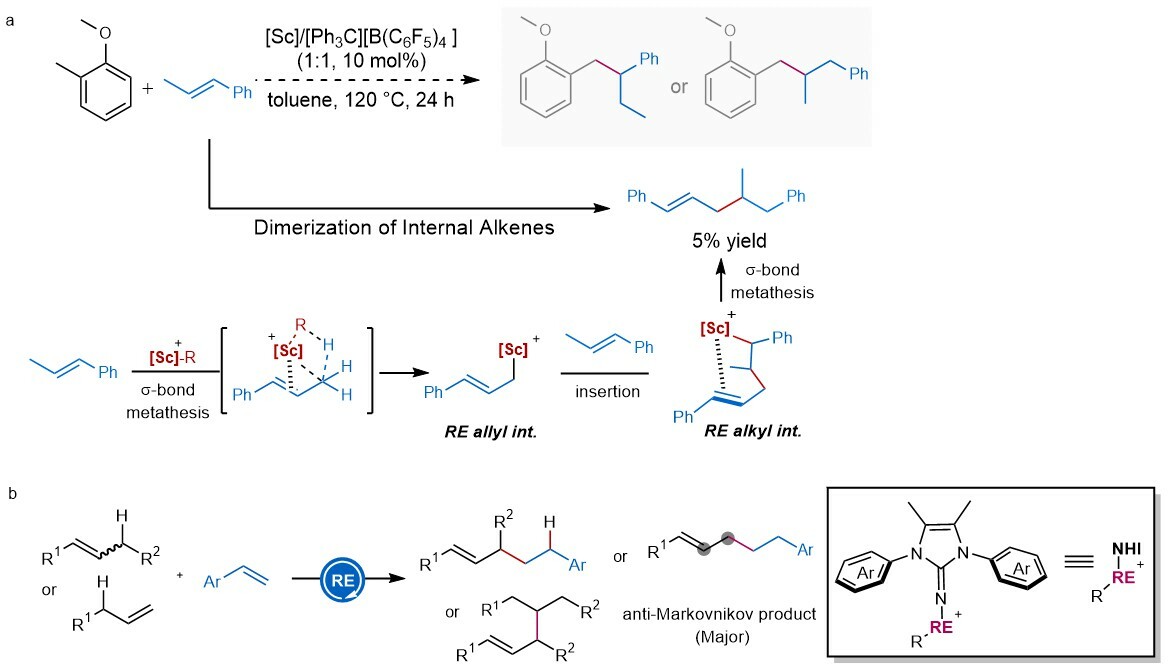
When we carried out the reaction of 2-methylanisoles with β-methylstyrene in the presence of scandium(III) alkyl complexes, dimerization product of β-methylstyrene was isolated in ca. 5% yield rather than the expected C–H alkylation of 2-methylanisole (Figure 2a). The interesting mechanism of the above reaction tempers us to further explore the related reaction, wherein the interaction between rare-earth metal ions and π-bonds of alkene triggers the C–H activation of allylic position (Figure 2b). Notably, compared with rare-earth (RE)…heteroatom interaction, RE...π interaction, frequently used in facilitating regio- and stereoselectivity of olefin polymerizations, is seldomly used to trigger catalytic C−H functionalization.
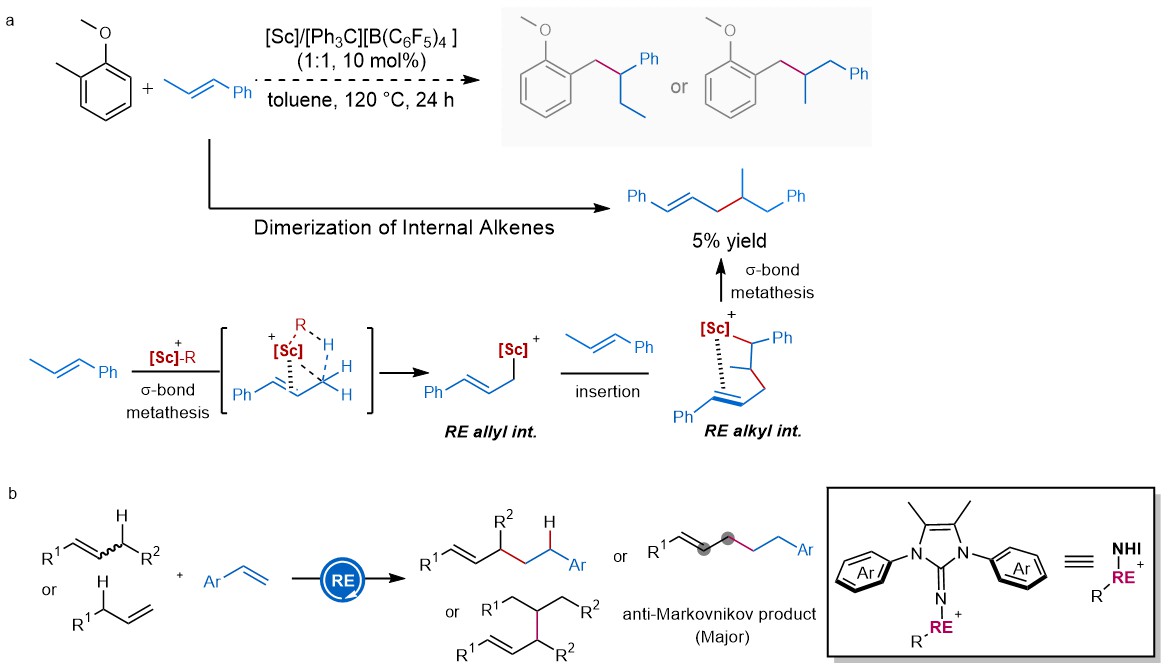
Figure 2. The allylic C-H functionalization of simple alkenes. a preliminary results on the reaction of 2-methylanisoles with β-methylstyrenes. b Rare-earth mediated direct hydroallylation of styrene derivatives with 1-aryl-2-alkyl alkenes and a-alkenes. Rare-earth (RE).
Challenges and Insights
There are several challenges associated with the above transformation: (1) the selective activation of allylic position through relatively weak RE…alkene interaction; (2) the influence of alkene configuration and highly fluxional behavior of allylic rare-earth metal intermediate on the reactivity and stereoselectivity; and (3) the control of hydroallylation of two different alkenes instead of dimerization or polymerization. In this work, cationic imidazolin-2-iminato (NHI) scandium(III) alkyl complexes were identified to be efficient promoters for the anti-Markovnikov-type hydroallylation of styrenes with alkenes as well as dimerization of internal and terminal alkenes.
Impact and Practical Implication
Taking use of the interplays of rare-earth metal with C=C double bond, we successfully achieved hydroallylation of styrene derivatives with internal and terminal alkenes as well as dimerization of alkenes by cationic imidazolin-2-iminato scandium alkyl complexes. A variety of chain elongated internal alkenes were obtained in an atom-economical and efficient manner with moderate to good yields and high E/Z ratio. A possible catalytic cycle involving the assistance of Lewis base was proposed to understand the reaction mechanism and E/Z ratio of hydroallylation reaction. This work provides a new protocol for the direct hydroallylation of alkenes with alkenes. The development of chiral imidazolin-2-iminato rare-earth alkyl complexes and their further utility in other related C‒H functionalization reactions are in progress.
References
- Nishiura, M.; Guo, F.; Hou, Z. Chem. Res. 2015, 48, 2209.
- Kundu,; Inoue, M.; Nagae, H.; Tsurugi, H.; Mashima, K. J. Am. Chem. Soc. 2018, 140, 7332.
- Gao, H. J.; Su, J. H.; Xu, P. F.; Xu, X. Chem. Front. 2018, 5, 59.
- Lin, H. L.; Li, Y. R.; Wang, J. Y.; Zhang, M.; Jiang, T.; Li, J.; Chen, Y. H. Appl Organomet Chem 2021, 35, e6345.
- (a) Wu, X.; Tamm, M. Chem. Rev. 2014, 260, 116. (b) Ochiai, T.; Franz, D.; Inoue, S. Chem. Soc. Rev. 2016, 45, 6327. (c) Revathi, S.; Raja, P.; Saha, S.; Eisen, M. S.; Ghatak, T. Chem. Commun. 2021, 57, 5483.
- (a) Li, D. W.; Ning, L. C.; Luo, Q. L.; Wang, S. Y.; Feng, X. M.; Dong, S. X. China Chem. 2023, 66, 1804. (b) Wang. S. Y.; Zhu, C. H.; Ning, L. C.; Li, D. W.; Feng, X. M.; Dong, S. X. Chem. Sci. 2023, 14, 3132. (c) Li, D.; Wang, Y. J.; Wang, S. Y.; Dong, S. X. Chem. Eur. J. 2024, e202401014.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in