Atg7 senses ATP levels to promote survival during metabolic stress
Published in Cell & Molecular Biology

Background:
Atg7 plays a crucial role in maintaining intracellular homeostasis through its interactions with Atg8 and Atg12. Our previous studies identified a role for Atg7, independent of its E1-like enzymatic activity, in regulating the transcription of the cell cycle inhibitor p21 by binding to the tumor suppressor p53. In the absence of Atg7, prolonged metabolic stress leads to increased DNA damage and p53-dependent apoptosis. Our lab also studied the role of Atg7 in the Warburg effect. Atg7 inhibits the Warburg effect by binding to and preventing excessive phosphorylation of PKM2. A deficiency of Atg7 leads to hyperphosphorylated PKM2, inducing the Warburg effect and promoting the epithelial-mesenchymal transition.
About the Study:
The above studies confirmed the mechanisms by which Atg7 maintains energy homeostasis, including the sources and pathways of energy. To further investigate the role of Atg7 in energy consumption, we utilized mass spectrometry analysis to identify interacting partners. Among numerous candidates, we specifically focused on Programmed Cell Death 4 (PDCD4), which acts as an inhibitor of protein translation and aligns with our interest in studying mechanisms that reduce energy consumption. We therefore focused on the functional interaction between Atg7 and PDCD4.
As an E1-like activating enzyme, Atg7 shares the same ATP-binding domain as other E1-like enzymes. Simulating conditions of ATP deficiency by introducing 2-deoxyglucose and oligomycin to inhibit glycolysis and ATP synthesis led us to discover the potential of Atg7 to sense ATP. Initially, we considered Atg7 to be primarily a carrier for ATP, providing ATP for the binding of AKT (protein kinase B) to PDCD4. We believed that Atg7 could only function in the presence of ATP and not in its absence. In this regard, Atg7 would behave more like a sensor or switch, performing its function when the signal is turned on.
Due to technical limitations, our study did not directly demonstrate structural changes in Atg7 in response to ATP, or any alterations in protein modifications typically observed upon contact with ATP. Additionally, a review of relevant literature did not find any protein modifications, such as phosphorylation, ubiquitination, or acetylation, that are associated with Atg7 function. However, these findings suggested a new direction for our research efforts. In this study, we focused on validating the ATP-sensing ability of Atg7 from a functional perspective. We expressed wild-type or inactive forms of Atg7 in Atg7-KO cells and then observed the dynamics of the downstream AKT-PDCD4 interaction. This allowed us to determine that Atg7 can sense ATP.
In summary, our findings reveal that Atg7 can interact with different proteins to regulate energy homeostasis. The ATP-sensing ability of Atg7 positions it as a checkpoint in energy metabolism, providing a new perspective for studying Atg7 in the context of energy homeostasis.
Key Findings:
For the first time, we propose that Atg7 can function as an ATP sensor. We validated this concept by regulating downstream protein binding. Additionally, we discovered a new role of Atg7 in maintaining energy metabolism homeostasis, specifically by reducing protein translation and lowering energy consumption.
Through the Atg7-AKT-PDCD4 phosphorylation-ubiquitination axis, we demonstrated the dynamic transmission of stress signals in cells experiencing ATP depletion. Under normal conditions, the adenylated domain on Atg7 detects ATP and facilitates AKT phosphorylation of PDCD4. This makes PDCD4 more susceptible to phosphorylation and degradation by the proteasome, resulting in lower levels of PDCD4. However, during starvation, Atg7 senses a scarcity of ATP that leads to reduced interaction with PDCD4, thereby decreasing its degradation. The elevated level of PDCD4 then functions to inhibit protein translation and reduce energy consumption, enabling cells to survive starvation. These findings suggest that the Atg7-AKT-PDCD4 axis serves as a metabolic adaptation pathway that helps cells overcome metabolic stress.
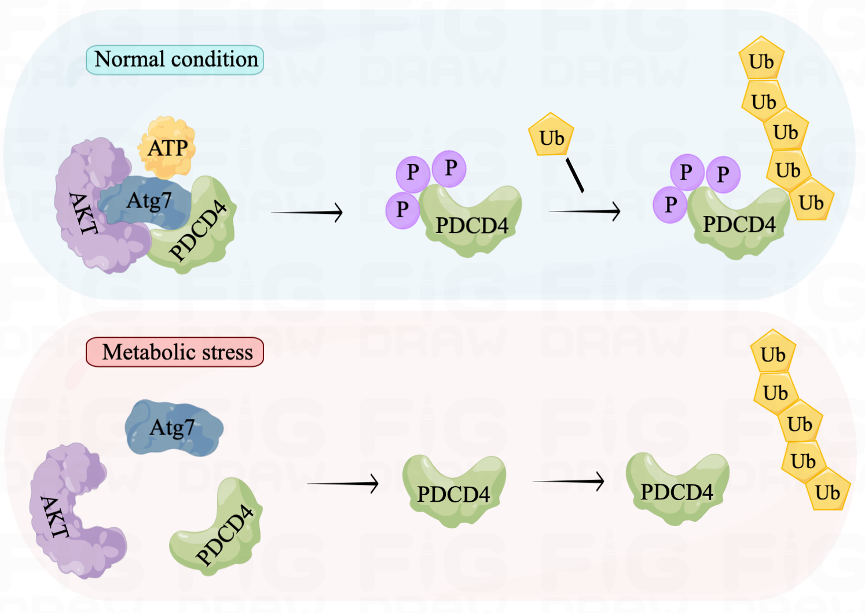
Figure 1. Atg7-AKT-PDCD4 axis in the cellular metabolic homeostasis
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in