Directly imaging emergence of phase separation in peroxidized lipid membranes
Published in Chemistry
Lipid peroxidation is instrumental in regulating the cell’s metabolism, inflammation, immune response or cell death;1–4 and it is thought to be involved in the development of diseases including atherosclerosis,5 cancer6 or Alzheimer’s.7 In addition, this process is exploited in photodynamic therapy to selectively cause the apoptosis of malignant cells.8 Yet, despite its relevance in biology, the effect of lipid peroxidation on the structure and biophysical properties of lipid membranes is a subject of debate.
Oxidation of phospholipid molecules, which make up cellular membranes, involves the reaction of carbon-carbon double bond(s) in the lipid tails with reactive oxygen species, ROS. In particular, the reaction with singlet oxygen leads to the creation of a lipid hydroperoxide, Fig. 1a. Here we show that the presence of the -OOH moiety leads to significant alterations in the membrane’s structural and biophysical properties, by combining optical microscopy, X-ray diffraction and molecular dynamics (MD) simulations.
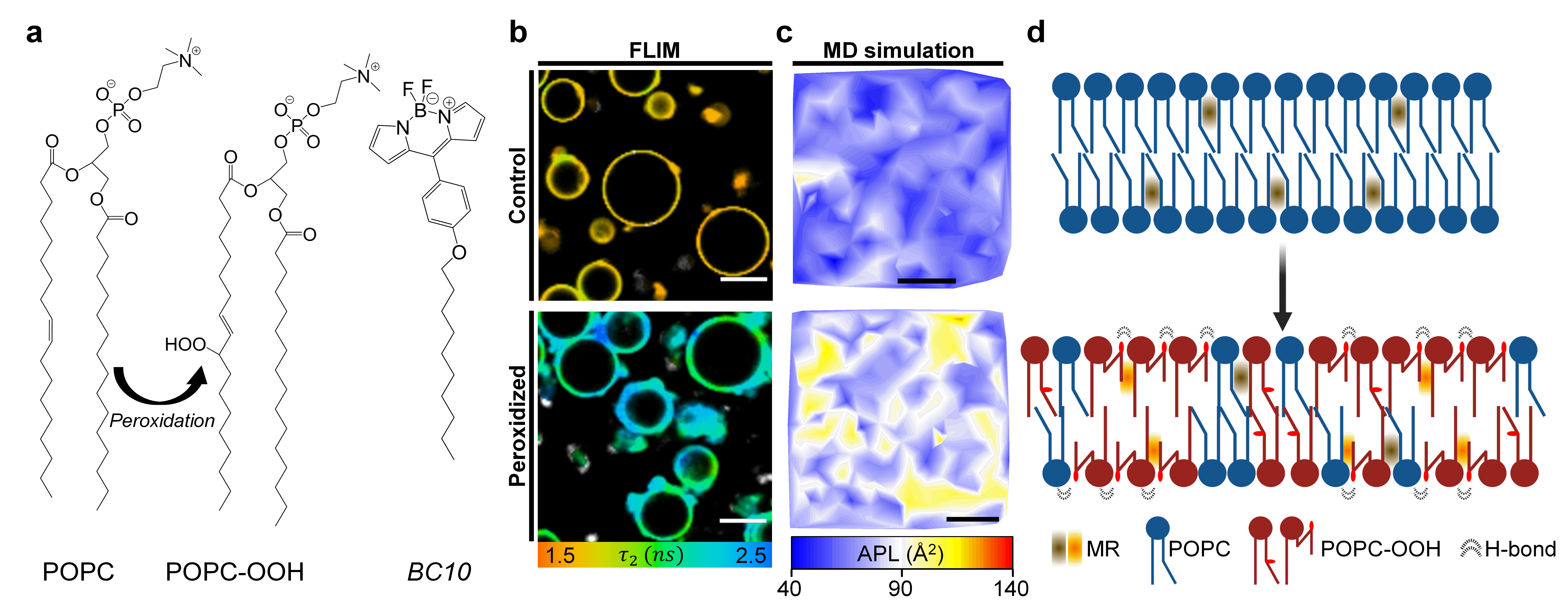
Figure 1. a, Molecular structure of the lipids and molecular rotor BC10 used in this work. More viscous environment will result in an increased lifetime from BC10 b, Fluorescence Lifetime Imaging Microscopy (FLIM) revealed an increase in the membrane’s viscosity and lateral heterogeneity in the presence of lipid peroxides. c, MD simulations confirmed the formation of lipid clusters with distinct molecular configuration, here exemplified by changes in the area per lipid (APL). d, A schematic showing the effect of lipid peroxidation on the membrane’s architecture.
We first formed synthetic liposomes containing an increasing amount of the peroxidized lipid POPC-OOH and stained them using an environmentally sensitive fluorophore, termed molecular rotor (MR). The intramolecular rotation of these molecules depends on their surrounding microviscosity – in our case, the degree of lipid packing – and this is reflected in the viscosity-dependent change in the probe’s fluorescence emission intensity and lifetime.10 Importantly, fluorescence lifetime is independent of the probe’s concentration, the excitation setup, or effects such as photobleaching, and this allows, after an appropriate calibration, to directly quantify the viscosity in the vicinity of the MR. Using a BODIPY-based molecular rotor (BC1011, Fig. 1a; or its charged analogue12), we measured a 50% increase in membrane viscosity from non-oxidized to fully peroxidized membranes. This change was similar in magnitude to the increase observed during in-situ peroxidation experiments, where singlet oxygen was generated in situ, by mimicking photodynamic therapy cancer treatment. These results suggested that the presence of -OOH motifs did not disrupt the in-plane organization of the lipid bilayers, and instead caused increased the molecular ordering within the membrane.
To further investigate this phenomenon, we examined lipid stacks containing an increased fraction of peroxidized lipid using small- and wide-angle X-ray diffraction (SAXS/WAXS). The diffraction patterns suggested that the presence of lipid peroxides had two surprising effects: i) The membranes became floppier (i.e. their thermal fluctuations were enhanced) and ii) the lateral heterogeneity of the bilayer increased. The higher membrane elasticity was also confirmed through flickering spectroscopy, a technique based on imaging the membrane’s thermal fluctuations. The concurrent increase in microviscosity and decrease in bending rigidity of peroxidized membranes contrasted with that of archetypical membranes (e.g. POPC without peroxidation), where both quantities are directly correlated.
On the other hand, our second observation indicated a larger variance in the area occupied by each lipid molecule after peroxidation; a behaviour which could be compatible with membranes displaying regions with distinct degrees of lipid packing, yet this hypothesis is in apparent contradiction with Gibb’s rule. To further explore this idea, we stained giant unilamellar vesicles (GUVs) with the MR and used fluorescence lifetime imaging microscopy (FLIM) to obtain quantitative maps of the membrane’s viscosity (Fig. 1b). By examining these images, we were able to detect a significantly wider microviscosity distribution in GUVs containing the lipid peroxide – even in those composed solely of the peroxidized molecules – which gave further evidence for domain formation. Altogether, this suggests that the presence of lipid peroxidation products can promote the appearance of lipid clusters of higher local order, which could potentially impact signal transduction and the mechanical behaviour of the cell’s membrane.
Finally, we applied all-atom (AA) and molecular-dynamics (MD) simulations in order to gain further insight into our experimental observations. Here, the key finding was that polar -OOH group from the peroxidized lipid tail was able to migrate towards the membrane interface but, crucially, only around 40% of the oxidized chains displayed this behaviour. As a consequence membrane thickness decreased, and so did the bending modulus, hence explaining the observed increase in the bilayer’s thermal fluctuations in both SAXS and flickering spectroscopy experiments. In addition, snorkelling of the peroxidized lipid species implies that there are, effectively, the equivalent of two different molecular species (e.g. with the -OOH group either embedded within the hydrocarbon region or snorkelling towards the membrane’s surface); and this would explain how lipid single-component membranes could display lateral heterogeneity. In fact, snapshots of our simulations showed a homogeneous area per lipid (APL) when membranes were composed of unoxidized lipids; while those containing 100% of the peroxidized molecules showed marked regions where the APL was higher or lower (Fig. 1c). By examining lipid-lipid contact maps, we discovered a significant interaction between the oxygen atoms in the oxidized -OOH tail of the lipids, as well as between these oxygens and the oxygen atoms in the ester groups of lipid heads. This is indicative of the formation of hydrogen bonds between these groups and could serve as a mechanism for the formation of lipid clusters observed both in silico and experimentally.
In summary, we showed that the presence of -OOH containing alkyl chains causes the snorkelling of the lipid tail towards the lipid-water interface. This disrupts the canonical correlation between the membrane elasticity and viscosity and leads to the emergence of more ordered lipid regions in single-component membranes, as depicted in Fig. 1d. Overall, our results highlight the drastic effects of oxidative stress on the biophysical behaviour of biomembranes, and we anticipate these changes will play a key role in controlling the cellular response and in the progression of diseases including Alzheimer’s or atherosclerosis.
- Gaschler, M. M. & Stockwell, B. R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482, 419–425 (2017).
- Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. 113, E4966–E4975 (2016).
- Hajeyah, A. A., Griffiths, W. J., Wang, Y., Finch, A. J. & O’Donnell, V. B. The Biosynthesis of Enzymatically Oxidized Lipids. Front. Endocrinol. (Lausanne). 11, 1–32 (2020).
- Katikaneni, A. et al. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 22, 1049–1055 (2020).
- Berliner, J. A., Leitinger, N. & Tsimikas, S. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 50, S207–S212 (2009).
- Clemente, S. M., Martínez-Costa, O. H., Monsalve, M. & Samhan-Arias, A. K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 25, 5144 (2020).
- Butterfield, D. A. Brain lipid peroxidation and alzheimer disease: Synergy between the Butterfield and Mattson laboratories. Ageing Res. Rev. 64, 101049 (2020).
- Dos Santos, A. F. et al. Distinct photo-oxidation-induced cell death pathways lead to selective killing of human breast cancer cells. Cell Death Dis. 11, 1070 (2020).
- Itri, R., Junqueira, H. C., Mertins, O. & Baptista, M. S. Membrane changes under oxidative stress: The impact of oxidized lipids. Biophys. Rev. 6, 47–61 (2014).
- Kuimova, M. K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 14, 12671–12686 (2012).
- Dent, M. R. et al. Imaging phase separation in model lipid membranes through the use of BODIPY based molecular rotors. Phys. Chem. Chem. Phys. 17, 18393–18402 (2015).
- López-Duarte, I., Vu, T. T., Izquierdo, M. A., Bull, J. A. & Kuimova, M. K. A molecular rotor for measuring viscosity in plasma membranes of live cells. Chem. Commun. 50, 5282–5284 (2014).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in