Divergent evolution of the human heart: What we can learn from our evolutionary cousins
Published in Ecology & Evolution and Anatomy & Physiology

Mammals are an extraordinary group of animals demonstrating vast biological diversity, ranging from the mighty blue whale (Balaenoptera musculus) that rules our oceans, to the diminutive fennec fox (Vulpes zerda) native to the harsh Sahara Desert. The ability of mammals to thrive across the world happened over many millennia by evolutionary adaptation, a process that selects physiological traits that favor survival and reproductive success. Whilst adaptation has influenced many aspects of physiology, such as the respiratory and immune systems, it was previously suggested to have bypassed the heart, leaving it highly conserved among mammals1.
Previous research from our group, however, suggested that the structure of the human heart may be different from that of our closest evolutionary relative, the chimpanzee (Pan troglodytes)2. In adult male chimpanzees, the left ventricle - which receives oxygenated blood from the lungs and pumps it around the body - contains bundles of muscles that extend into the chamber cavity, called trabeculations. Whereas, the left ventricle of the human heart has a comparatively smoother ventricular wall.
We became particularly excited by this finding, and wanted to examine whether left ventricular trabeculations were also common among the other great apes. And if so, why might humans be the odd one out?
Trabeculation: A normal phenotype among non-human great apes
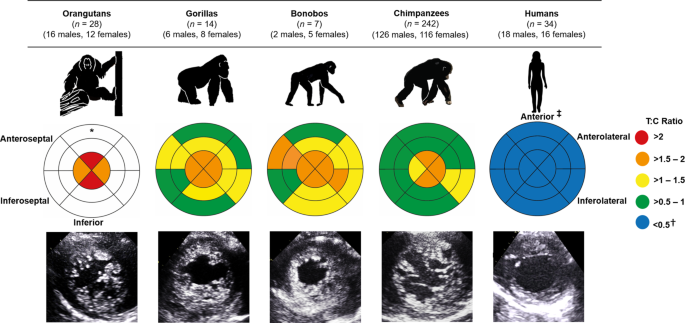
Over six years, in collaboration with a remarkable team of dedicated veterinary and care professionals from four sanctuaries and five zoos across Africa, Asia and Europe, we created a large cardiac dataset covering all extant great apes. Using ultrasound, we examined and compared the structure and function of the left ventricle across 242 chimpanzees, 7 bonobos (Pan paniscus), 14 gorillas (Gorilla gorilla), 28 orangutans (Pongo) and 34 humans. In contrast to the comparatively smoother ventricular wall typically observed in humans, we identified a more trabeculated ventricular myocardium across all non-human great apes, regardless of age or sex. This difference was particularly noticeable at the apex, the bottom of the left ventricle, where the difference between humans and great apes was approximately four-fold (Fig 1).
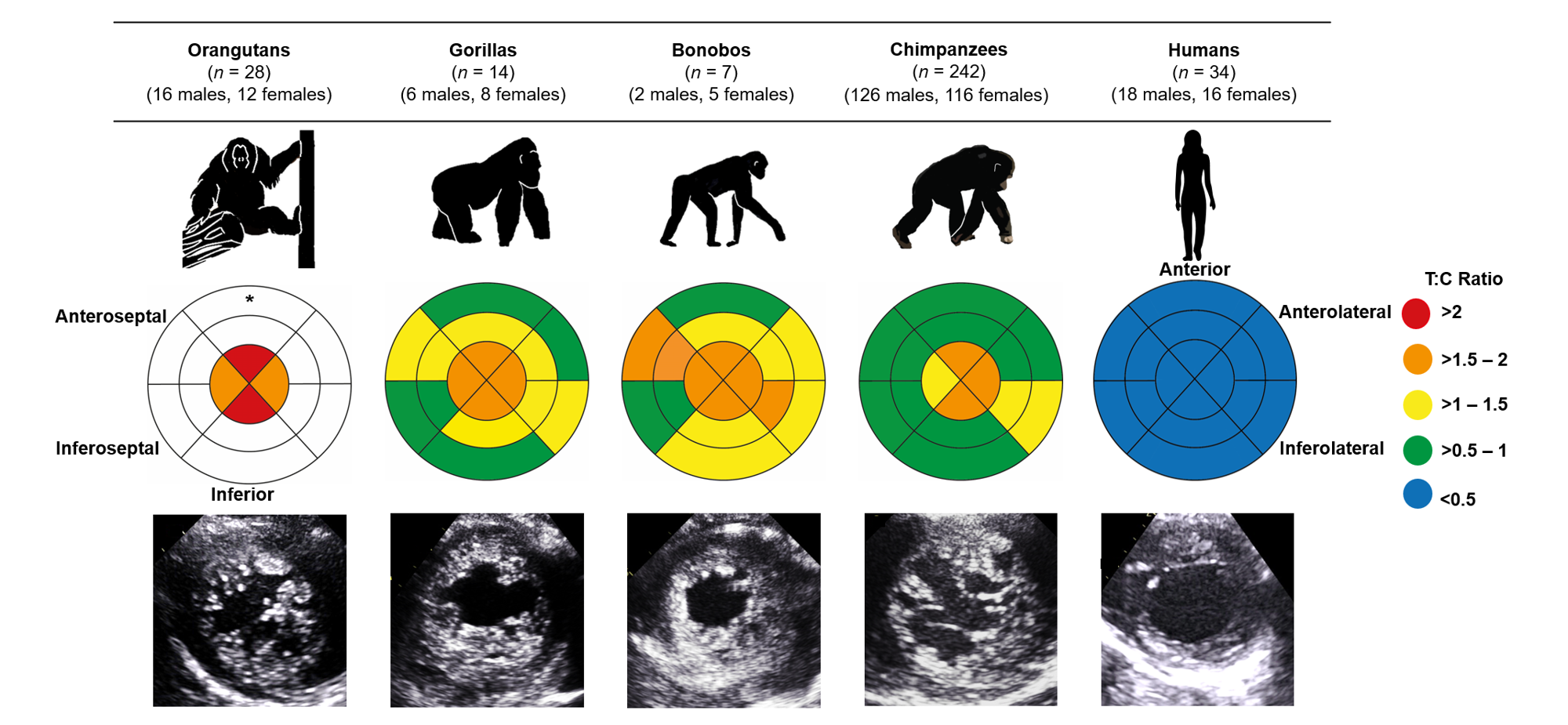
Figure 1. Comparison of left ventricular trabeculation in great apes. The bullseye plots represent the trabecular:compact (T:C) ratio for each segment of the left ventricle. A greater T:C ratio reflects a higher degree of trabeculation. The outer layer of the bullseye plots represents the basal segments, the middle and innermost layers represent the midpapillary and apical segments of the left ventricle, respectively. Echocardiographic images of the parasternal short-axis at the apex are shown at end-diastole. *No data were available for the basal or midpapillary segments in the orangutans due to artefact from laryngeal air sacs.
Functional implications of trabeculation?
As form and function are often closely linked, we were interested as to whether the differences in structure between the human and non-human great ape heart coincided with differences in function. We used a technique called speckle-tracking echocardiography, which traces the pattern of the cardiac tissue throughout the cardiac cycle, to examine the deformation, rotation and twisting of the left ventricle. Our findings indicated a negative curvilinear relationship between the degree of trabeculation and the amount of twist and rotation at the apex during contraction. In other words, humans, which have the least trabeculation, have much greater rotation of the apex and twisting of the left ventricle, whereas non-human great apes, which have much greater trabeculation, have less rotation and twist (Fig 2).
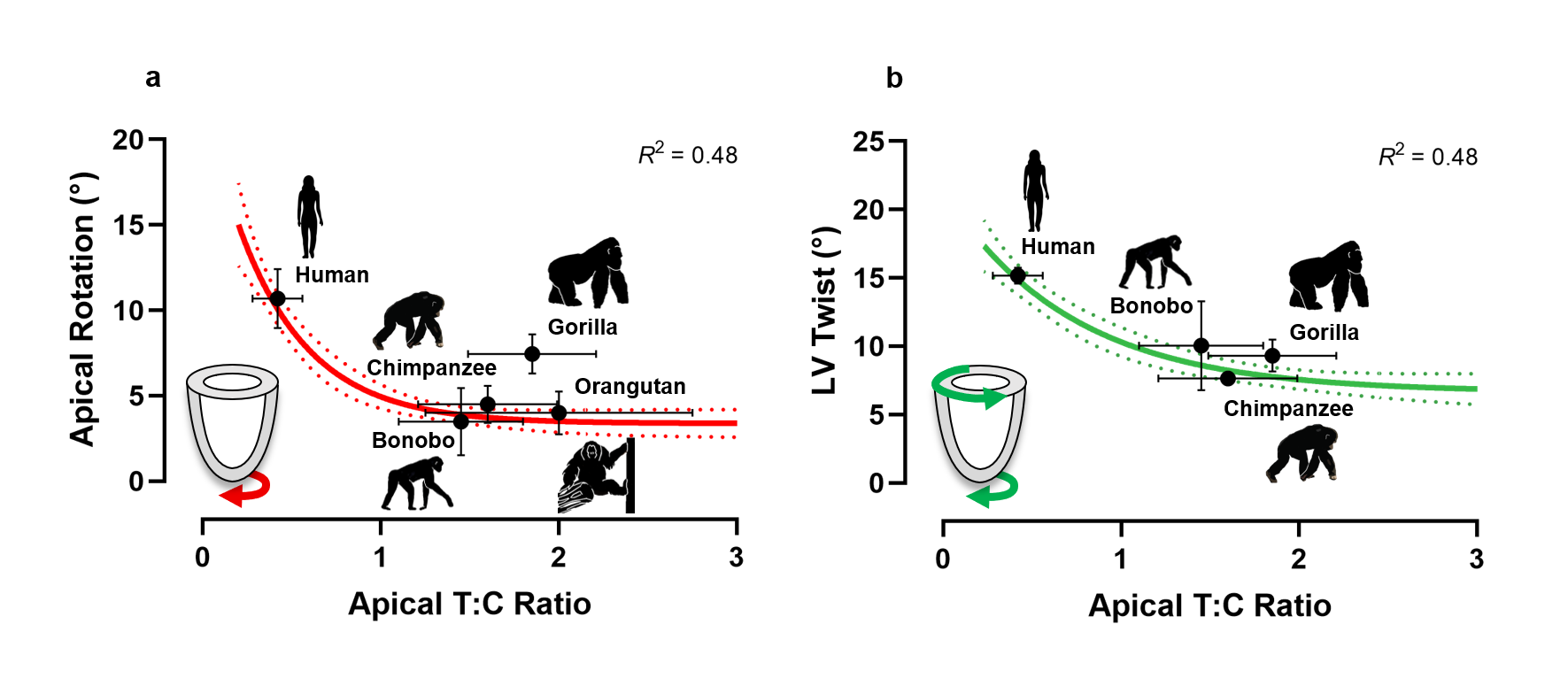
Figure 2. Relationship between markers of left ventricular (LV) function and apical trabeculation in great apes. (a) Peak apical rotation, shown in red, and (b) peak LV twist during contraction, shown in green. Analyses of LV twist was not possible in any of the orangutans due to artefact from laryngeal air sacs. The exponential plateau curve is shown, with the 95% confidence bands represented by the dotted line. The mean and standard error are shown in black for each species.
Divergent evolution of the human heart
Since human’s divergence from our last common ancestor with chimpanzees ~8 – 6 million years ago, our brain underwent a considerable increase in size and we became bipedal, engaging in greater amounts of daily activity. These are associated with a much larger metabolic cost and greater thermoregulatory stress, which may have placed selective pressure on the heart to adapt. The evolutionary divergence of the human left ventricle away from a trabeculated myocardium, towards a comparatively smoother ventricular wall may have facilitated greater cardiac deformation, rotation and twisting. In turn, this could result in a larger volume of blood to be ejected from the heart to meet the augmented metabolic and thermoregulatory demands of the human environment. Overall, these data suggest that rather than being bypassed by evolutionary adaptation as previously suggested1, there are a number of subtle differences between the hearts of closely related mammalian species; and that these may reflect the selective pressures of distinct environments.
Working with our evolutionary cousins
While all data collection has its challenges, working with great apes in remote locations can be particularly tricky! It required an enormous amount of preparation beforehand to ensure we had all the necessary equipment and medical consumables for every animal. Once we arrived, we were often in remote geographical locations (think long car journeys on unpaved roads with minimal suspension, and rocky boat rides along the river with expensive equipment; Fig 3), facing extreme environmental conditions (we can confidently say we have never seen torrential downpours quite as bad as those during the rainy season in Congo!). However, the logistics of coordinating the research and care teams (often across three continents), the establishment of productive relationships with all stakeholders, and the transportation of the equipment and supplies were only made possible through the combined efforts of a large (and fantastic) interdisciplinary team. Through this combined approach, we have had the honor of working with our closest evolutionary cousins, many of which are recognized as critically endangered.
Importantly, whilst our research is focused on the evolution of the human heart, we are also heavily invested in the healthcare of these incredible species. In captive great apes, cardiac disease is a leading cause of death. However, before our work with these species, little was known about their normal cardiovascular physiology. Together with veterinary practitioners, our work through practical workshops and the establishment of normative data 3–7 have also improved the understanding, diagnosis and management of heart disease in great apes.

References
- Meijler, F. L. & Meijler, T. D. Archetype, adaptation and the mammalian heart. Neth Heart J 19, 142–148 (2011).
- Shave, R. E. et al. Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proc Natl Acad Sci U S A 116, 19905–19910 (2019).
- Drane, A. L. et al. Cardiac structure and function characterized across age groups and between sexes in healthy wild-born captive chimpanzees (Pan troglodytes) living in sanctuaries. Am J Vet Res 80, 547–557 (2019).
- Drane, A. L. et al. THE INFLUENCE OF ANESTHESIA WITH AND WITHOUT MEDETOMIDINE ON CARDIAC STRUCTURE AND FUNCTION IN SANCTUARY CAPTIVE CHIMPANZEES (PAN TROGLODYTES). J Zoo Wildl Med 52, 986–996 (2021).
- Drane, A. L., Shave, R., Routh, A. & Barbon, A. An exploratory investigation of echocardiographic parameters and the effects of posture on cardiac structure and function in the Livingstone’s fruit bat (Pteropus livingstonii). Veterinary Radiology & Ultrasound 59, 89–97 (2018).
- Atencia, R. et al. HEART RATE AND INDIRECT BLOOD PRESSURE RESPONSES TO FOUR DIFFERENT FIELD ANESTHETIC PROTOCOLS IN WILD-BORN CAPTIVE CHIMPANZEES (PAN TROGLODYTES). J Zoo Wildl Med 48, 636–644 (2017).
- Drane, A. L. et al. Evaluation of relationships between results of electrocardiography and echocardiography in 341 chimpanzees (Pan troglodytes). Am J Vet Res 81, 488–498 (2020).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Nitric oxide signalling in cardiovascular health and disease Hub
A new Communities’ space to connect, collaborate, and explore research on Cardiovascular Physiology, Clinical Medicine, and Diseases!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in