Epichaperomics: Illuminating the Secrets of Protein Interactions in Disease Research
Published in Cancer, Protocols & Methods, and Cell & Molecular Biology
Background: In the vast realm of biomedical research, scientists are constantly striving to unravel the mysteries underlying diseases and develop effective treatments. A recent breakthrough in the field has led to the introduction of an innovative platform called epichaperomics. This cutting-edge technology enables researchers to delve deep into the intricate world of protein interactions, providing valuable insights into disease mechanisms and opening new avenues for therapeutic interventions. Traditionally, scientists studied diseases by looking at mutations, genes, transcripts and protein levels. However, recent research has shown that studying how thousands of proteins interact with each other across different cells and tissues provides a better understanding of diseases. Epichaperomics focuses on these protein interactions, which hold the key to disease mechanisms.
Understanding the Power of Protein Networks: Proteins, the workhorses of our cells, rarely act alone. They engage in intricate interactions, forming networks that drive crucial cellular functions. These protein-protein interaction (PPI) networks play a vital role in determining the characteristics and behavior of cells and organisms. By examining these networks, scientists gain a better understanding of how diseases disrupt normal cellular processes and impact overall health.
The Epichaperomics Approach: However, until now, it has been challenging to examine these thousands of protein interactions comprehensively and in their natural state. Enter special chemicals binding to protein structures that are involved in faulty protein networks associated with diseases, called epichaperomes. Researchers have ingeniously leveraged the power of epichaperomes and epichaperome binders to develop the epichaperomics platform, a leap forward in the study of PPI dysfunctions in disease (Figure 1).
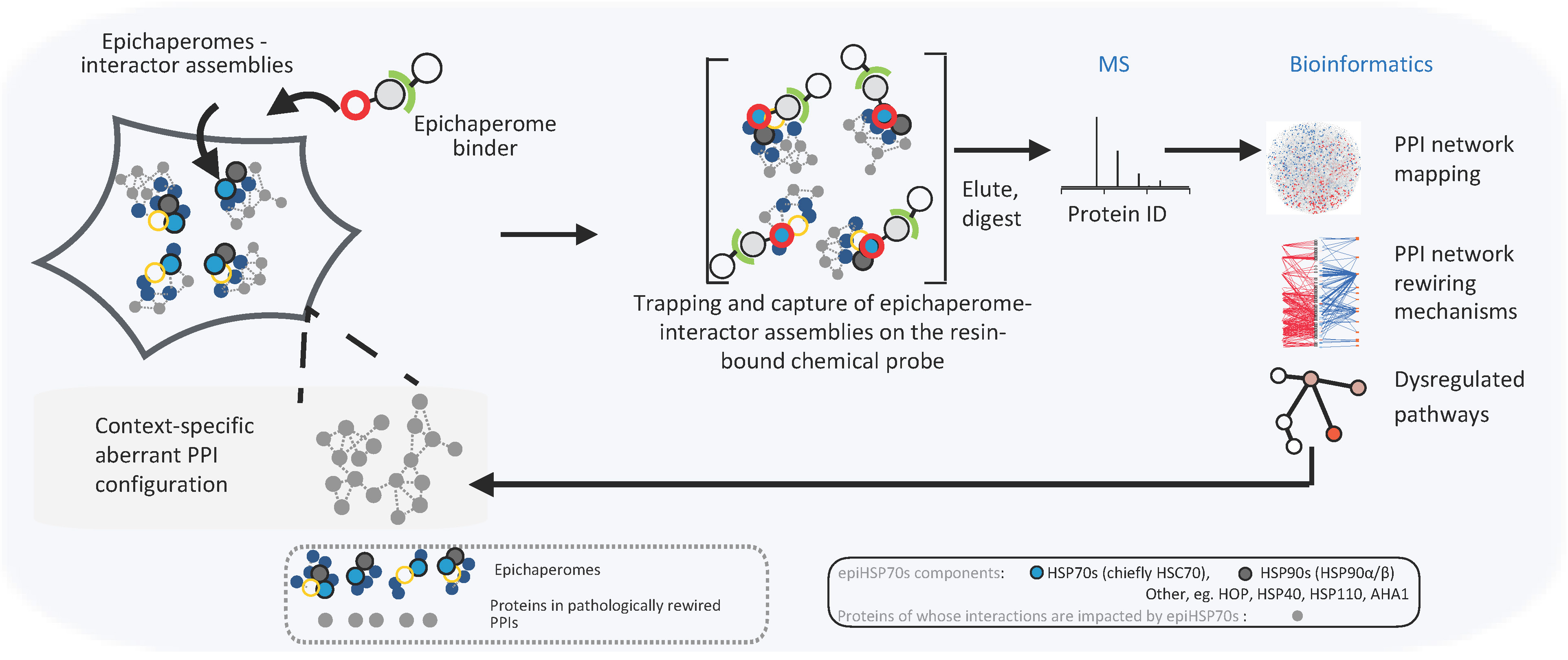
Unraveling Disease Mechanisms with Epichaperomics: Through the application of epichaperomics, researchers have made strides in unraveling disease mechanisms. By identifying and analyzing context-specific changes in PPIs, they have gained invaluable insights into various diseases, including cancer and Alzheimer’s disease. In a proof-of-principle study published in Nature Communications, they applied this method to cancer cells, mapping the changes in interactors among thousands of proteins involved in cancer-related processes. Through epichaperomics they found a mechanism in cancer cells that enhances the activity of proteins involved in cell division. This finding provides valuable insights into how tumors grow and suggests new ways to target cancer cells. They applied the platform to Alzheimer's disease (AD) brain specimens and AD-related cellular and mouse models to show how stressors associated with Alzheimer's disease rewire brain networks leading to synaptic dysfunction and memory loss. Epichaperomics thus offers researchers a powerful tool to explore the complex web of protein interactions in their native context, revealing fundamental insights that could transform our understanding of diseases and pave the way for innovative therapeutic solutions.
Promising Therapeutic Strategies: One of the most exciting aspects of epichaperomics is its potential as a therapeutic strategy. By pinpointing dysregulated protein interactions, scientists can develop targeted interventions aimed at restoring balance within PPI networks. These innovative approaches hold promise not only for cancer but also for other diseases, such as neurodegenerative disorders, cardiovascular conditions, and metabolic syndromes.
Conclusion: Epichaperomics by studying protein-protein interactions in their native environment is enabling scientists to penetrate the intricacies of disease mechanisms like never before. With its ability to identify, analyze, and modulate PPI dysfunctions in their native disease state, this innovative platform holds tremendous promise for advancing biomedical research and developing targeted therapies. Epichaperomics is poised to unlock a wealth of knowledge, empowering scientists to decipher the secrets of diseases and make significant strides toward improving human health.
References:
- Ginsberg SD, Neubert TA, Sharma S, Digwal CS, Yan P, Timbus C, Wang T, Chiosis G. Disease-specific interactome alterations via epichaperomics: the case for Alzheimer's disease. FEBS J. 2022 Apr;289(8):2047-2066. doi: 10.1111/febs.16031. Epub 2021 Jun 12. PMID: 34028172; PMCID: PMC8611103.
- Rodina, A., Xu, C., Digwal, C.S. et al. Systems-level analyses of protein-protein interaction network dysfunctions via epichaperomics identify cancer-specific mechanisms of stress adaptation. Nat Commun 14, 3742 (2023). https://doi.org/10.1038/s41467-023-39241-7
- Inda MC, Joshi S, Wang T, Bolaender A, Gandu S, Koren Iii J, Che AY, Taldone T, Yan P, Sun W, Uddin M, Panchal P, Riolo M, Shah S, Barlas A, Xu K, Chan LYL, Gruzinova A, Kishinevsky S, Studer L, Fossati V, Noggle SA, White JR, de Stanchina E, Sequeira S, Anthoney KH, Steele JW, Manova-Todorova K, Patil S, Dunphy MP, Pillarsetty N, Pereira AC, Erdjument-Bromage H, Neubert TA, Rodina A, Ginsberg SD, De Marco Garcia N, Luo W, Chiosis G. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat Commun. 2020 Jan 16;11(1):319. doi: 10.1038/s41467-019-14082-5. PMID: 31949159; PMCID: PMC6965647.
- Ginsberg SD, Sharma S, Norton L, Chiosis G. Targeting stressor-induced dysfunctions in protein-protein interaction networks via epichaperomes. Trends Pharmacol Sci. 2023 Jan;44(1):20-33. doi: 10.1016/j.tips.2022.10.006. Epub 2022 Nov 20. PMID: 36414432; PMCID: PMC9789192.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in