Introduction
The most common subtype of lung cancer, non-small cell lung cancer (NSCLC), is characterized by a heterogeneous array of molecular aberrations that may drive tumor growth. For some of these alterations, such as EGFR mutations, therapies have been developed that can target the mutated EGFR protein and thereby impede tumor growth. By now, EGFR-targeted therapies are the standard-of-care for first-line treatment of metastatic EGFR-mutated NSCLC1. Besides EGFR mutations, both in NSCLC and other types of cancer, there are many other molecular aberrations for which targeted therapies are standardly available, available in compassionate use or off-label, or available in clinical trials2.
Among European patients with metastatic NSCLC, approximately 12-15% have an EGFR mutation and may be treated with EGFR-targeted therapy such as osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI). However, in addition to their EGFR mutation, many patients may simultaneously have other molecular aberrations. Examples of aberrations frequently co-occurring with an EGFR mutation are mutations in TP53, PIK3CA, APC and CTNNB1 and copy number alterations of CDK6, BRAF, CDK4 and MYC.3 The presence of certain co-occurring aberrations can affect the expected efficacy of specific treatment options and may warrant considering treatment options other than the standard-of-care. For example, the co-occurrence of an EGFR mutation and a TP53 mutation in metastatic NSCLC is associated with shorter duration of response to first-line osimertinib, the current standard-of-care4. Meanwhile, the addition of ramucirumab (a monoclonal antibody targeting VEGFR2) to treatment with erlotinib (a first-generation EGFR TKI) is associated with improved outcome in patients with EGFR-mutated NSCLC3. Therefore, patients with concurrent EGFR-mutated and TP53-mutated NSCLC may preferably be treated with combined EGFR TKI and ramucirumab (e.g., erlotinib + ramucirumab).
What did we report?
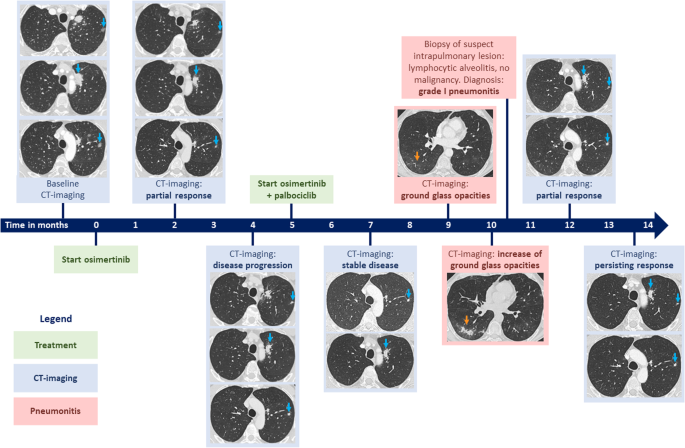
In our report, we described the case of a patient with metastatic NSCLC with an EGFR mutation, a PIK3CA mutation and CDK4 amplification, who was initially treated with osimertinib alone (standard-of-care). After a short response to osimertinib, the patient had progression of their disease. Subsequently, palbociclib (a CDK4/6 inhibitor) was added to osimertinib treatment, and at ten months of follow-up, the patient had a ongoing partial response.
Why does it matter?
Amplification of CDK4 or CDK6 occurs in approximately 10% of patients with EGFR-mutated NSCLC and is associated with shorter overall survival5. This suggests that CDK4/6 signaling may negatively affect the efficacy of EGFR inhibitors, which was also demonstrated in in vitro and in vivo models6. Together with the findings of our case report, patients with co-occurring EGFR mutation and CDK4/6 amplification may benefit from combined osimertinib and palbociclib treatment.
In our described case, palbociclib was prescribed off-label and combined with osimertinib. In general, prescribing targeted therapy in off-label setting or prescribing combinations of targeted therapies can be challenging7-9. Access to these therapies can be limited due to lack of reimbursement, lack of (nearby) clinical trials, trial ineligibility and lack of compassionate use programs. Specific to our case, though there have been clinical trials for CDK4/6 pathway-targeted therapy, trials typically do not allow for concurrent treatment with other (on-label) targeted therapies (e.g., osimertinib).
When to combine off-label and on-label targeted therapy?
The (potential) actionability of rare or complex molecular aberrations in patients with cancer can be discussed in Molecular Tumor Boards (MTBs). MTBs are multidisciplinary teams that discuss molecular tumor profiles and provide advice for optimal treatment of patients with cancer, in particular regarding options for targeted therapy. MTBs generally consist of at least (molecular) pathologists, pulmonary and/or medical oncologists, geneticists and (clinical) molecular biologists.10,11 While practices differ between countries and medical centers, MTBs are considered useful for treatment decision-making in patients with (advanced stage) cancer. We argue that, if advised by a Molecular Tumor Board, prescribing off-label targeted therapy in combination with on-label targeted therapy can be an effective treatment strategy and should therefore be reimbursed by insurance companies. However, patients should be (1) carefully monitored for therapy efficacy and potential toxicities/side effects and (2) periodically followed-up by the advising MTB. In other words, it is necessary for MTBs to evaluate their effectiveness and, if possible, treatment outcomes are registered in follow-up studies. Whenever possible, it is preferred that patients receive the advised treatment combination in an ongoing clinical trial.
References
1 Hendriks, L. E. et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34, 339-357 (2023). https://doi.org/10.1016/j.annonc.2022.12.009
2 de Jager, V. D. et al. Developments in predictive biomarker testing and targeted therapy in advanced stage non-small cell lung cancer and their application across European countries. Lancet Reg Health Eur 38, 100838 (2024). https://doi.org/10.1016/j.lanepe.2024.100838
3 Nishio, M. et al. RELAY, Ramucirumab Plus Erlotinib (RAM+ERL) in Untreated Metastatic EGFR-Mutant NSCLC (EGFR+ NSCLC): Association Between TP53 Status and Clinical Outcome. Clin Lung Cancer 24, 415-428 (2023). https://doi.org/10.1016/j.cllc.2023.02.010
4 Choudhury, N. J. et al. Molecular Biomarkers of Disease Outcomes and Mechanisms of Acquired Resistance to First-Line Osimertinib in Advanced EGFR-Mutant Lung Cancers. J Thorac Oncol 18, 463-475 (2023). https://doi.org/10.1016/j.jtho.2022.11.022
5 Sitthideatphaiboon, P. et al. Co-occurrence CDK4/6 amplification serves as biomarkers of de novo EGFR TKI resistance in sensitizing EGFR mutation non-small cell lung cancer. Sci Rep 12, 2167 (2022). https://doi.org/10.1038/s41598-022-06239-y
6 Hara, N. et al. CDK4/6 signaling attenuates the effect of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer. Transl Lung Cancer Res 12, 2098-2112 (2023). https://doi.org/10.21037/tlcr-23-99
7 von Itzstein, M. S. et al. Accessing Targeted Therapies: A Potential Roadblock to Implementing Precision Oncology? JCO Oncol Pract 17, e999-e1011 (2021). https://doi.org/10.1200/OP.20.00927
8 Peters, K. B. et al. Use, access, and initial outcomes of off-label ivosidenib in patients with IDH1 mutant glioma. Neurooncol Pract 11, 199-204 (2024). https://doi.org/10.1093/nop/npad068
9 Vancoppenolle, J. M. et al. Targeted combination therapies in oncology: Challenging regulatory frameworks designed for monotherapies in Europe. Drug Discov Today 28, 103620 (2023). https://doi.org/10.1016/j.drudis.2023.103620
10 Koopman, B. et al. Multicenter Comparison of Molecular Tumor Boards in The Netherlands: Definition, Composition, Methods, and Targeted Therapy Recommendations. Oncologist 26, e1347-e1358 (2021). https://doi.org/10.1002/onco.13580
11 Koopman, B. et al. Relevance and Effectiveness of Molecular Tumor Board Recommendations for Patients With Non-Small-Cell Lung Cancer With Rare or Complex Mutational Profiles. JCO Precis Oncol 4, 393-410 (2020). https://doi.org/10.1200/PO.20.00008
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in