Site Differentiation Strategy for Selective Strontium Uptake and Elution within an All-Inorganic Polyoxoniobate Framework
Published in Chemistry
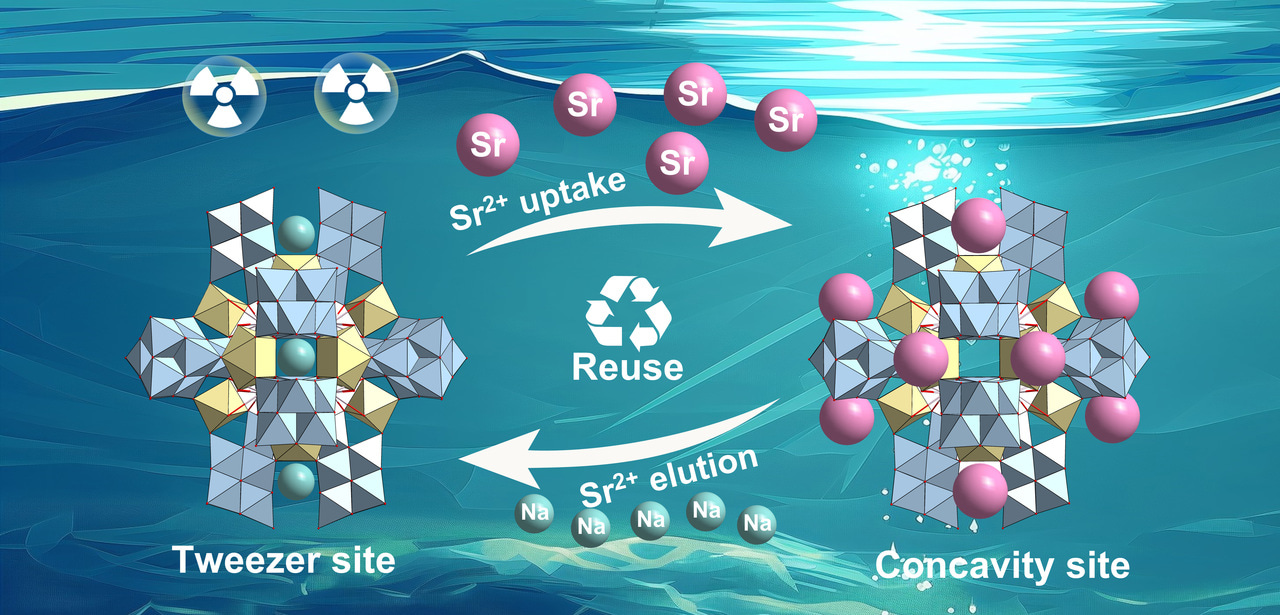
The rapid development of nuclear energy has created major challenges for the management of radioactive waste and remediation of contamination. Selective capture of trace amounts of hazardous radioactive 90Sr from large-scale high-level liquid waste (HLW) is crucial for sustainable development. Currently, besides the challenge of developing efficient and stable Sr2+ ion adsorbents, a more significant challenge lies in how to achieve the recycling of Sr2+ adsorbents for effectively treating large-scale HLW. Despite the emergence of various efficient inorganic 90Sr removers, including silicotitanates, titanates, layered metal chalcogenides, and layered metal oxides, to our knowledge, no 90Sr adsorbent has been able to achieve both high selectivity in capturing Sr2+ under high salt caustic conditions and easy elution and reuse. In this work, we made important progress as follows.
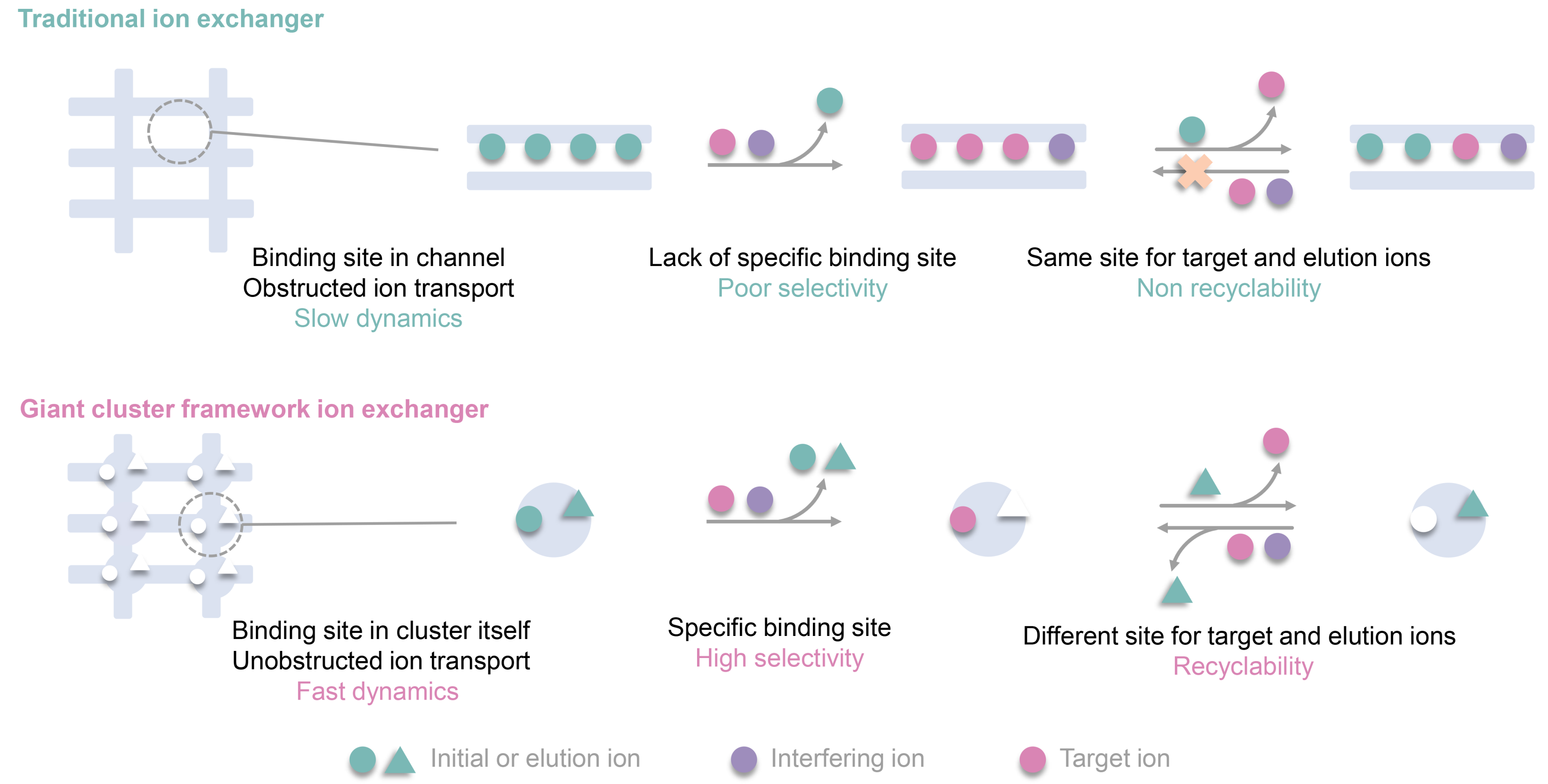
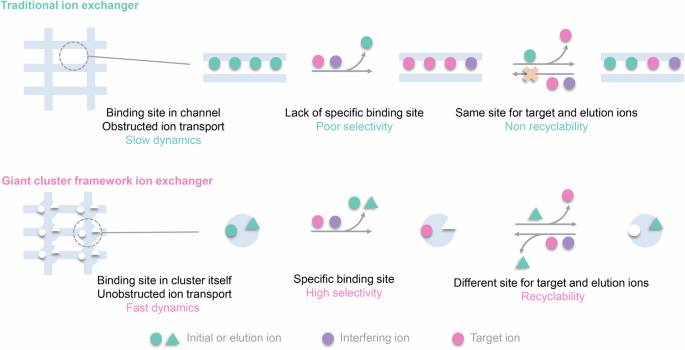
Propose a novel site differentiation strategy for Sr2+ uptake and elution. High selectivity and convenient elution are often contradictory. High selectivity means that the effective cavity diameter and chemical environment of the exchange active site will accommodate the target ions well, which makes it difficult for the captured ions to escape from this stable chemical environment. Generally, elution ions must overcome high binding energy to exchange target ions and replace the binding site, which is extremely challenging. Here, we propose a site differentiation strategy, that a Sr2+ adsorbent possesses two distinct ion adsorption sites, capable of selectively capturing target Sr ions and elution ions, respectively, it not only has the potential to efficiently adsorb Sr ions through the Sr2+ sites, but also can achieve efficient elution of Sr ions by loading elution ions onto the elution ion sites during the elution process, based on charge balance. It avoids the challenge faced by conventional Sr adsorbents where elution ions need to directly compete with Sr2+ ions for adsorption sites during elution.
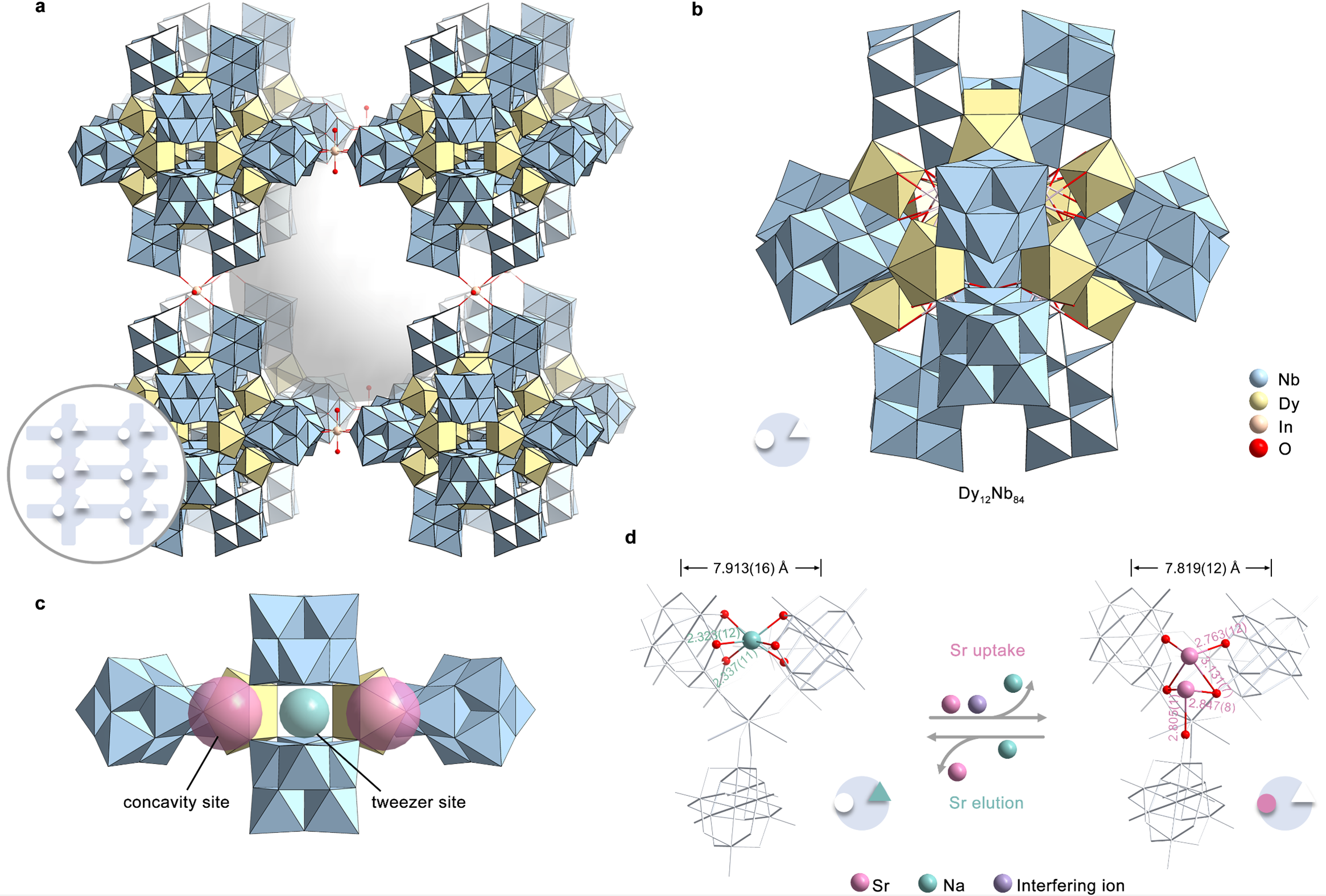
Demonstrate validity and reliability of the site differentiation strategy: an all-inorganic polyoxomolybdate (PONb) as a proof-of-concept model. Based on the concept of the site differentiation strategy, an all-inorganic giant PONb-based three-dimensional (3D) framework material (FZU-1) with the presence of distinct selective metal capture sites (concavity site and tweezer site) acts as a proof-of-concept ion exchanger. FZU-1 can not only effectively remove 98.9% of Sr²⁺ from simulated nuclear liquid waste, performing best among the reported Sr adsorbents, but also achieve desorption of adsorbed Sr²⁺ ions by selectively loading Na⁺ ions, thus enabling the recycling of FZU-1. Besides nuclear waste, FZU-1 can also be effectively used as a trace Sr²+ remover for ground-water remediation, capable of removing 98.6% of Sr²⁺ in a simulated groundwater with ppb level Sr²⁺ ions, performing nearly as effectively as excellent inorganic adsorbents. The exceptional stability of FZU-1, along with selective Sr²⁺ capture and elution capabilities, positions it as a promising candidate for large-scale nuclear waste treatment and groundwater remediation applications.
Unobstructed ion transport channels in FZU-1. Traditional inorganic metal ion exchangers with high-dimensional frame-work structures often encounter a common problem that limits their metal ion separation performance, namely, the initially trapped metal ions within the pores or interlayer spaces tend to block the transport channels for subsequent metal ion loading. FZU-1, a giant POM-based high-dimensional inorganic metal ion exchanger, are promising candidates to overcome this issue and exhibit excellent metal ion separation performance, as their metal-binding cavities are located on the giant POMs with unobstructed ion transport channels. The kinetics experiments show that the removal rate of Sr2+ rapidly reaches 90% within 5 min for the Sr uptake process, and leaching amount of Sr2+ reaches half of the total elution rate within 5 min for the Sr elution process. The rapid Sr²⁺ uptake and elution capabilities of FZU-1, positions it as an effective ion exchanger.
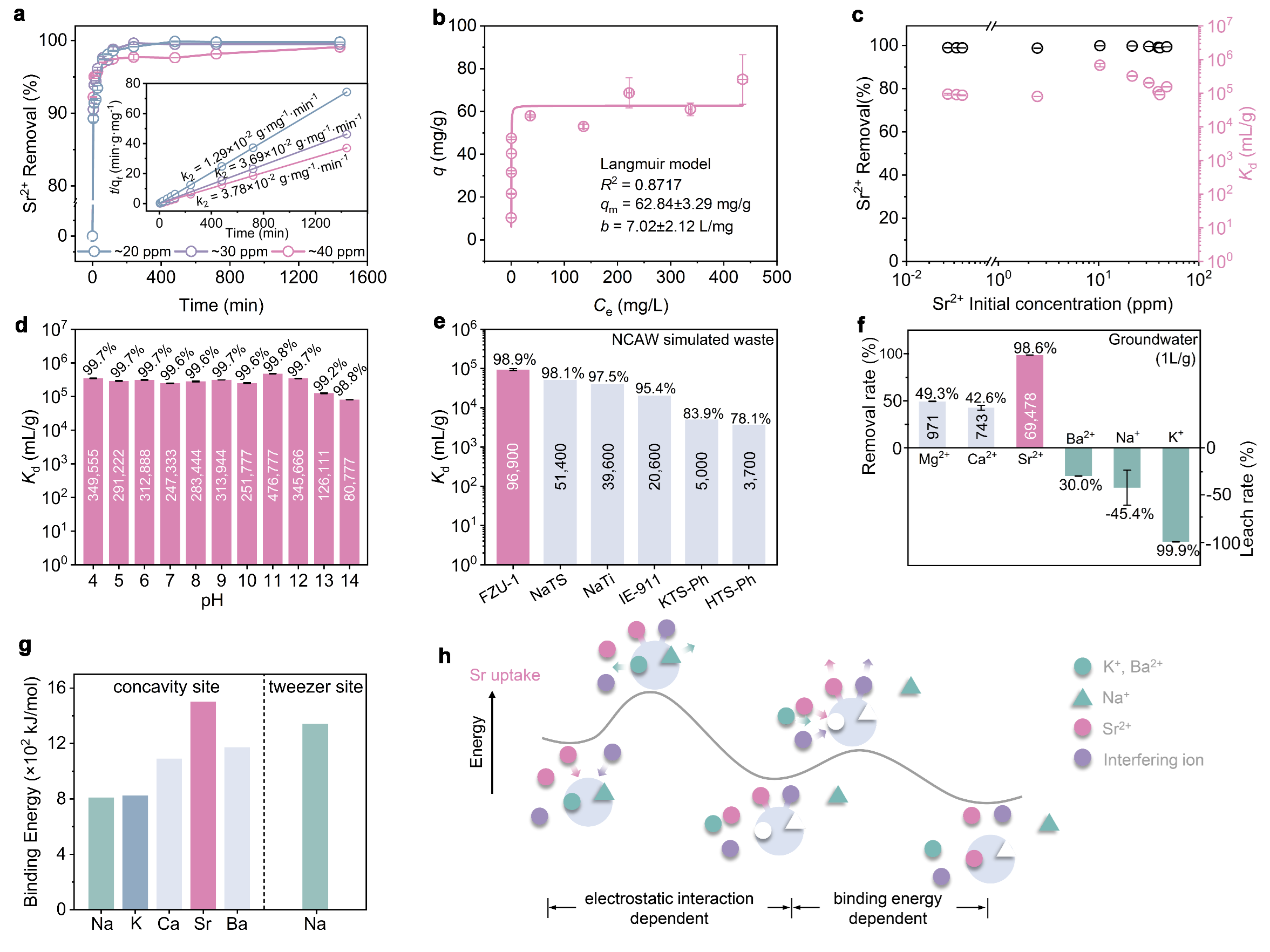
The well-defined structures during the Sr uptake and elution process endow us with atomic level insights into ion exchange mechanism. It is worth mentioning that most of reported Sr2+ absorbents are amorphous, polycrystalline, or unstable enough to capture Sr ions without well single crystal data. Even if the single crystal data can be determined, the location of the target ions can not be determined. Therefore, it is still a challenge to understand the ion exchange mechanism at the atomic level. Here, the high structural stability of FZU-1 enables its Sr2+ ion exchange to occur in a single-crystal-to-single-crystal manner while maintaining high crystallinity. A total of six sets of high-quality single-crystal X-ray diffraction data provides atomic-level insights into clarifying the selective Sr2+ uptake and elution mechanism for the first time. Experimental and theoretical calculations reveal that the FZU-1 efficiently entraps Sr2+ ions within the concavity sites of PONb subunits, while simultaneously leaching Na+ ions from the tweezer sites through the electrostatic potential generated by the captured Sr2+ ions. Conversely, during elution, the FZU-1Sr selectively captures Na+ ions at the tweezer sites, facilitating the efficient removal of Sr2+ ions from the concavity sites due to the additional positive charge imparted by the Na+ ions.
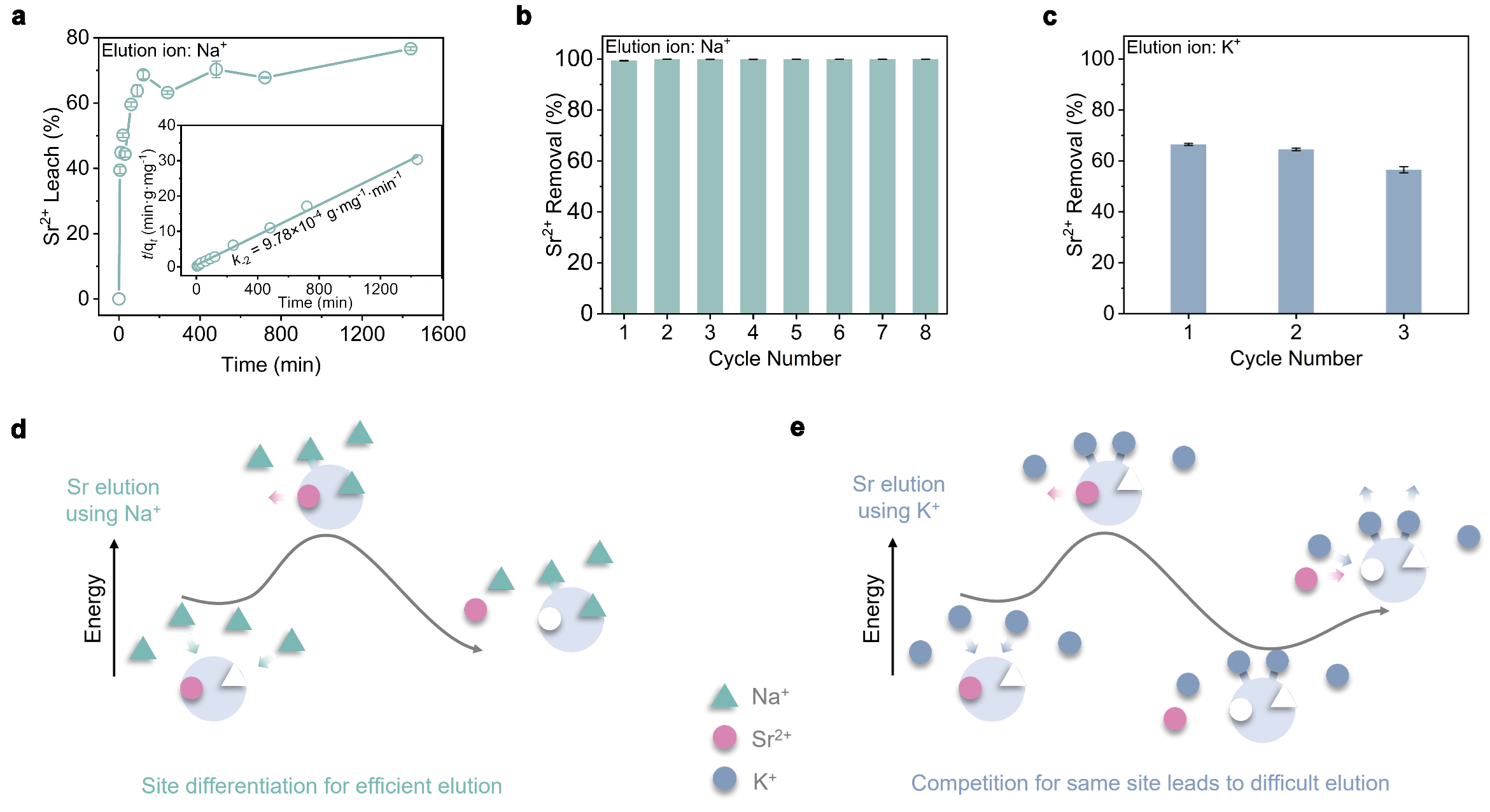
In summary, we have proposed for the first time the application of site differentiation strategy in ion exchange. FZU-1 efficiently captures Sr2+ ions from passing tank alkaline supernatant or contaminated groundwater through an ion exchange process, followed by subsequent liquid processing via established procedures. Unlike current adsorbents saturated with Sr2+, which necessitate awaiting uncertain disposal, a 3M Na+ solution can effectively elute Sr2+ from FZU-1, generating a concentrated, low-volume Sr-rich stream destined for the HLWs facility.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in