Targeting fatty acid synthase in preclinical models of triple-negative breast cancer brain metastases synergizes with chemotherapy and impairs invasion
Published in Cancer
Introduction/Background
Patients affected with brain metastases (BM) from breast cancer (BC) experience diminished quality of life, lower survival, and for a diversity of reasons (past exclusion from trials, poor CNS penetrance of some drugs, toxicity, etc) limited access to treatments with long-term benefits1-3. Patients with tumors that are human epidermal growth factor receptor 2 (HER2) positive and hormone receptor negative or triple-negative breast cancer (TNBC) subtypes have a greater risk of developing brain metastases. A recent study has shown that metastatic breast cancer cells need to acquire the ability to produce new fatty acids to more effectively colonize the brain, due to the low supply of fatty acids into brain tissue from the blood stream4.
In this study, we focus on inhibiting fatty acid synthase (FASN), an enzyme that produces fatty acids, in TNBC BM. We utilized cell lines with ability to metastasize to the brain, including two novel cell lines generated in our lab from TNBC BM tumors serially resected 6-months apart from the same patient at the University of Michigan Health System.
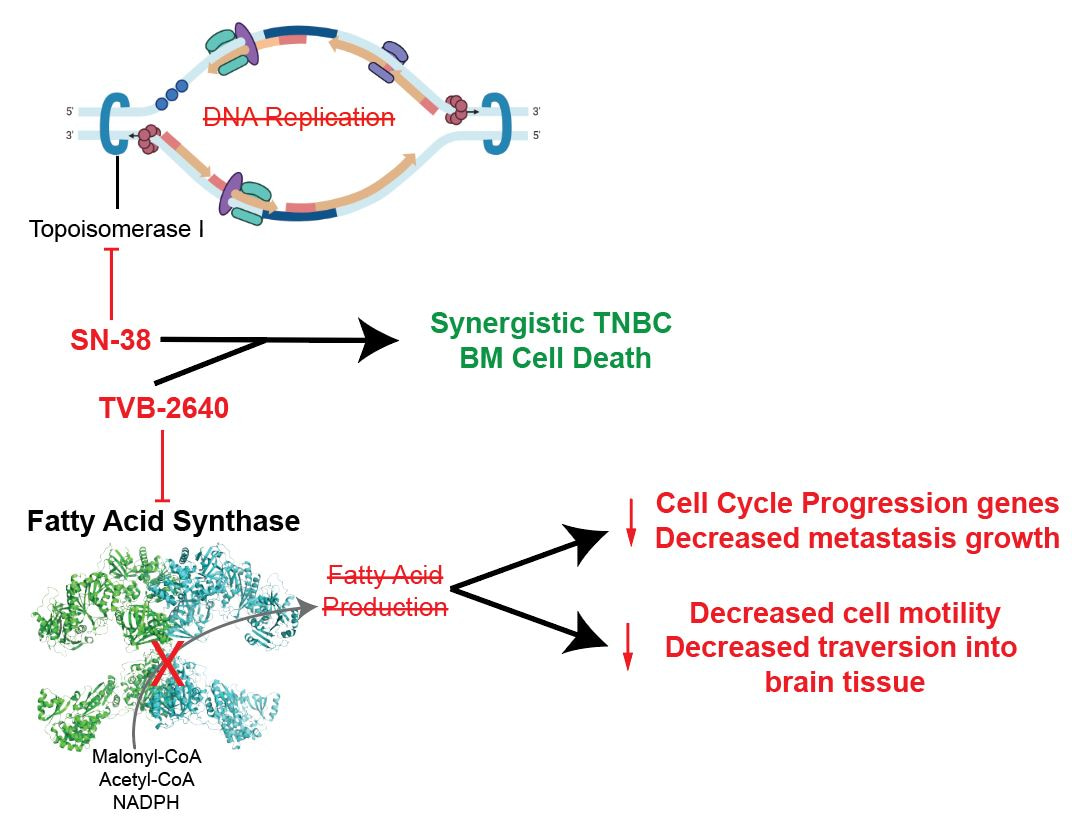
Major Findings
- We first showed that FASN inhibition with a FASN inhibitor synergizes with a chemotherapy that is already used in TNBC. We combined FASN inhibitors with chemotherapy to treat TNBC BM cell lines in 3D culture, while restricting their access to lipids. We evaluated synergy of the FASN inhibitor and chemotherapy combination, meaning we assessed whether the drugs kill many more cancer cells together than they do individually.5,6 The combinations were synergistic regardless of lipid availability, despite FASN inhibitors having a minimal ability to kill cells alone.
- We next validated that FASN inhibitors do in fact engage with the FASN pathway. We treated TNBC BM cell lines with FASN inhibitor or control in 3D culture and restricted access to lipids for 5 days. We collected RNA and rapidly profiled the expression of 768 metabolism-related genes. We then analyzed what genes increased or decreased expression with FASN inhibition. We found that 3 key genes involved in fatty acid synthesis had significant increases in expression level with FASN inhibition. This demonstrates that the cancer cells attempted to upregulate the expression of fatty acid synthesis genes to compensate for the FASN inhibition, verifying target engagement of FASN inhibitors. Additionally, we found the cells treated with a FASN inhibitor had significant decreases in the expression of genes involved in cell division and DNA repair, thus showing that the level of inhibition was functionally important to inhibit typical behaviors of aggressive cancer cells.
- We found that FASN inhibition leads to observable suppression of cell migration and invasion. Based on the results of 2, we wanted to understand whether the functional changes induced by FASN inhibition could affect metastatic behavior. We tested the outgrowth and invasion of spheroids treated with FASN inhibitors or control and noted significantly decreased spheroid-outgrowth in all TNBC BM cell lines following FASN inhibition. We then validated this suppressed cell migration using invasion and wound healing assays. Migration/invasion was significantly reduced following inhibition of FASN in TNBC BM cell lines. Based on these findings, we hypothesize that FASN is not only important for the establishment of brain metastases, but also for initiating invasion and metastasis.
Conclusion
This study introduces the idea of pairing already approved chemotherapy with potent FASN inhibition in TNBC BM. Our work shows that not only does FASN inhibition increase the efficacy of chemotherapy, but it also reduces the ability of cells to metastasize. FASN inhibitors are currently being evaluated in the clinic for treatment of nonalcoholic fatty liver disease and several types of cancer, including HER2+ breast cancer (NCT03179904). Our work provides rationale to further explore FASN inhibition as a therapeutic strategy specifically in TNBC BMs, where there is a dire need for new strategies to improve survival. Studies in animal models of TNBC BM are needed as the next step to translate these findings to the clinic.
References
1 Mills, M. N. et al. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat 180, 279-300, doi:10.1007/s10549-020-05552-2 (2020).
2 Hosonaga, M., Saya, H. & Arima, Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev 39, 711-720, doi:10.1007/s10555-020-09881-y (2020).
3 Kadamkulam Syriac, A., Nandu, N. S. & Leone, J. P. Central Nervous System Metastases from Triple-Negative Breast Cancer: Current Treatments and Future Prospective. Breast Cancer (Dove Med Press) 14, 1-13, doi:10.2147/BCTT.S274514 (2022).
4 Ferraro, G. B. et al. Fatty Acid Synthesis Is Required for Breast Cancer Brain Metastasis. Nat Cancer 2, 414-428, doi:10.1038/s43018-021-00183-y (2021).
5 Chou, T. C. & Talalay, P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22, 27-55, doi:10.1016/0065-2571(84)90007-4 (1984).
6 Chou, T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58, 621-681, doi:10.1124/pr.58.3.10 (2006).
Follow the Topic
-
npj Breast Cancer

This journal publishes original research articles, reviews, brief communications, matters arising, meeting reports and hypothesis generating observations which could be unexplained or preliminary findings from experiments, novel ideas or the framing of new questions that need to be solved.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Molecular Tumor Board in Breast Cancer
Publishing Model: Open Access
Deadline: Jul 22, 2026
Rare breast cancer subtypes
Publishing Model: Open Access
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in