Targeting Stem Cells in Medulloblastoma
Published in Cancer and Neuroscience
The clinical problem:
Medulloblastoma is an aggressive brain tumor that predominantly affects children. Many patients are cured with the current standards-of-care, but these therapies fail in 10-20% of patients. In all four medulloblastoma subgroups, recurrence remains a problem. Treatment fails when CSCs survive radiation and chemotherapy and regrow the tumor, causing recurrence. New approaches are needed that can specifically target this CSC population to disrupt recurrence.
The tumor biology problem:
Prior studies have identified and characterized CSCs in medulloblastoma patients and mouse models [1-7]. In both SHH and Group 3 subgroups, prior studies have shown that oligodendrocyte transcription factor 2 (OLIG2) plays a key role in tumor recurrence [8, 9] (Figure 1). These studies raise the possibility that OLIG2 may perform an essential tumor-promoting function and could be disrupted to prolong patient survival.
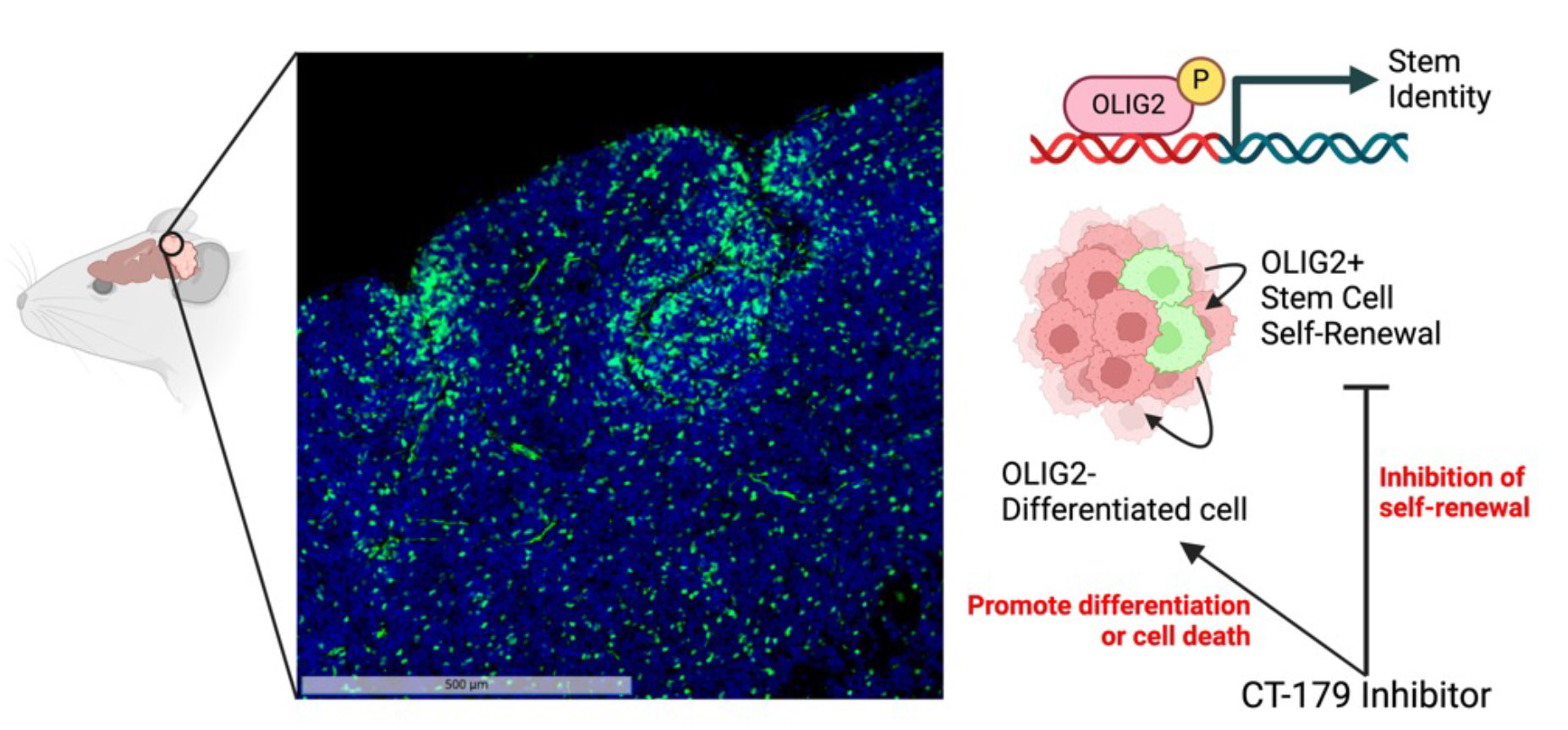
Figure 1: Transcription factor OLIG2 as a targetable regulator of stemness. In a mouse model of medulloblastoma, OLIG2+ stem cells are immunostained green, with all nuclei labeled blue (DAPI). These OLIG2+ cells divide slowly to renew their population and to generate rapidly proliferative daughter cells that contribute to the bulk of the tumor. We hypothesized that OLIG2 not only marks the identity of these cells but also controls their tumorigenesis, and that inhibiting OLIG2 would reduce their tendency to generate active tumor cells.
Created with BioRender.com
A team effort:
In work published concurrently with our study, a team led by Dr. Kinjal Desai and Prof. Peter Dirks (University of Toronto) show that OLIG2 switches medulloblastoma stem cells from a dormant quiescent state to an actively proliferative state (https://www.nature.com/articles/s41467-024-54858-y.) [10]. In a parallel, international collaboration including researchers from Sweden, Canada, Australia and the United States, we tested the therapeutic potential of OLIG2 inhibition using CT-179, a novel brain penetrant small molecule developed by Curtana Pharmaceuticals, designed to block OLIG2 transcriptional regulation. In this study, we tested CT-179 using three distinct models: transgenic mice engineered to develop medulloblastoma, mice implanted with medulloblastoma tumor cells and explant organoids derived from freshly resected medulloblastoma tumor tissue. The latter explant organoid approach is currently the state-of-the-art in 3D solid tumor culture systems, retaining tumor heterogeneity, immune infiltrate and vitally the tumour microenvironment (TME) (Figure 2). Together, we were able to leverage these powerful translational approaches for new insights into OLIG2 biology and CT-179 efficacy.
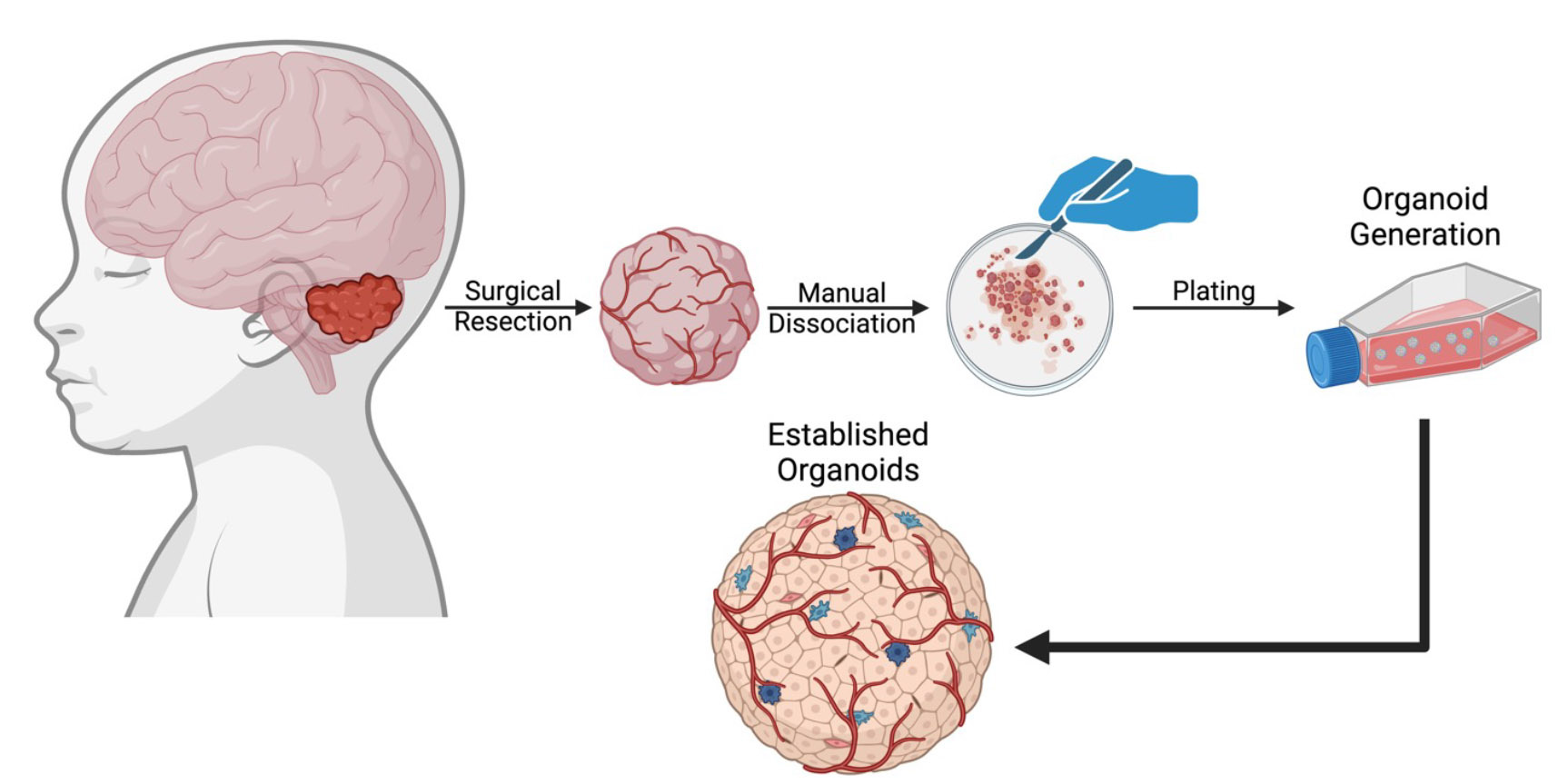 Figure 2: Medulloblastoma organoids generated from fresh patient tumor tissue retaining tumor heterogeneity. Medulloblastoma organoids were generated directly from fresh tumor tissue surgically resected from patients, without cell dissociation. These organoids have heterogeneous morphology, vascular structures, and proliferative and stem cell populations.
Figure 2: Medulloblastoma organoids generated from fresh patient tumor tissue retaining tumor heterogeneity. Medulloblastoma organoids were generated directly from fresh tumor tissue surgically resected from patients, without cell dissociation. These organoids have heterogeneous morphology, vascular structures, and proliferative and stem cell populations.
Created with BioRender.com
Understanding CT-179 specificity and efficacy:
OLIG2 has been shown to significantly correlate with outcome in SHH-MB patients [8, 9]. To investigate OLIG2 function in medulloblastoma, we silenced OLIG2 using small interfering RNAs (siRNA) in MB cell line. Our results showed OLIG2 knockdown (KD) disrupted cell cycle progression and induced apoptosis. Two key challenges to targeting OLIG2 function were finding a potent brain-penetrant inhibitor and also to show impact on CSCs. Unlike enzymes, transcription factors are notoriously hard to inhibit pharmacologically [11, 12]. OLIG2, like other basic helix-loop-helix (bHLH) transcription factors must dimerize to be active [13, 14]. CT-179 was designed to block OLIG2 function preventing dimerization [15, 16]. To test CT-179 on-target specificity we collaborated with Drs. Vukojevic, Oasa and Terenius from the Karolinska Institute in Sweden. Here the team employed fluorescence cross-correlation spectroscopy (FCCS) to show that CT-179 specifically blocks OLIG2 dimerization in live cells.
In vivo, CT-179 slowed tumor growth in each model system tested. To examine if this response was directly attributed to OLIG2+ CSCs, we developed a protocol to track CSC proliferation during CT-179 therapy. To achieve this, we labeled all actively dividing cells with BrdU prior to therapy, administered Edu to label dividing cells post-therapy, and used flow cytometry to identify OLIG2+ CSCs. This approach allowed us to assess whether OLIG2+ stem cells dividing before therapy decreased proliferation during treatment (Figure 3). This BrdU/Edu double labeling technique established that CT-179 effectively reduced the division frequency of OLIG2+ stem cells.
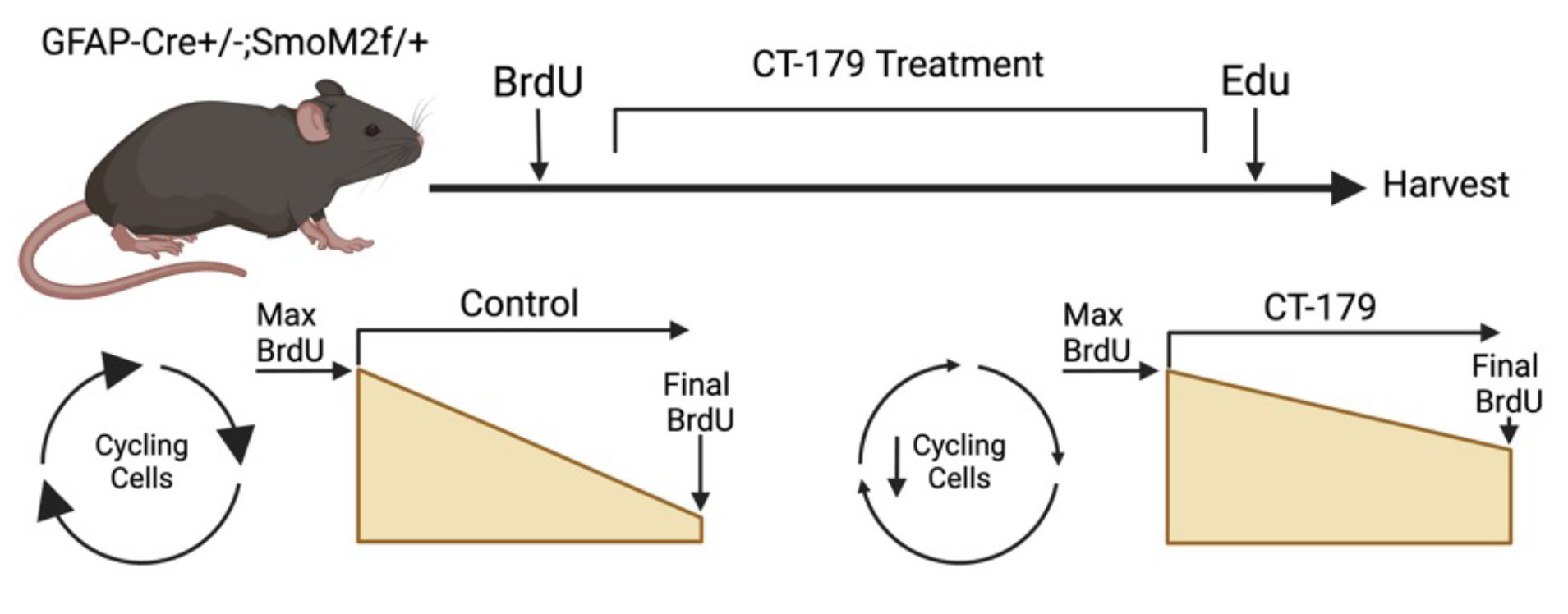 Figure 3: Labeling tumors with BrdU and Edu DNA binding agents permits investigation of cell cycling over the course of treatment with CT-179. In a GFAP-Cre/SmoM2 mouse model of medulloblastoma (MB) driven by a mutant Smoothened allele, constitutive SHH-pathway activation results in spontaneous medulloblastomas that resemble patient tumors. BrdU administered prior to treatment labels all cycling cells. Administered at the end of treatment, EdU marks cells still proliferating. OLIG2 marks CSCs and oligodendrocytes, and SOX10 distinguishes oligodendrocytes from CSCs.
Figure 3: Labeling tumors with BrdU and Edu DNA binding agents permits investigation of cell cycling over the course of treatment with CT-179. In a GFAP-Cre/SmoM2 mouse model of medulloblastoma (MB) driven by a mutant Smoothened allele, constitutive SHH-pathway activation results in spontaneous medulloblastomas that resemble patient tumors. BrdU administered prior to treatment labels all cycling cells. Administered at the end of treatment, EdU marks cells still proliferating. OLIG2 marks CSCs and oligodendrocytes, and SOX10 distinguishes oligodendrocytes from CSCs.
Created with BioRender.com
Overcoming treatment resistance:
We hypothesized that CT-179 alone would not induce a robust anti-tumor response in vivo. CT-179 targets only the tumor stem cell pool and in clinical practice, medulloblastoma always requires combination therapy. Transcriptomic analysis with single-cell resolution, scRNA-seq, allowed us to specifically analyze the proliferative tumor cell fraction during CT-179 therapy. Our scRNA-seq studies showed that cells that remained proliferative after CT-179 therapy up-regulated cyclin-dependent kinase 4 (CDK4), suggesting that inhibiting CDK4 might enhance the anti-tumor response to CT-179. This insight guided our final strategy: targeting CSCs with CT-179 while also simultaneously inhibiting CDK4 with the FDA-approved agent palbociclib. This combination of agents further prolonged survival. Leveraging transcriptomics following single agent therapy, enabled us to develop a clinically viable alternative to the harsh standards-of-care such as radiation and chemotherapy. Treatments like CT-179 combined with palbociclib offer hope for children suffering from medulloblastoma. The current standards-of-care are highly toxic and often leave patients with debilitating therapeutic sequelae. Our study provides a novel framework for investigating and targeting the CSC niche in heterogeneous solid tumors, while advancing the potential use of targeted brain penetrant small-molecule inhibitors, such as CT-179, in future CNS tumor-directed clinical trials.
References
- Selvadurai, H.J., et al., Medulloblastoma Arises from the Persistence of a Rare and Transient Sox2(+) Granule Neuron Precursor. Cell Rep, 2020. 31(2): p. 107511.
- Vladoiu, M.C., et al., Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature, 2019. 572(7767): p. 67-73.
- Ocasio, J., et al., scRNA-seq in medulloblastoma shows cellular heterogeneity and lineage expansion support resistance to SHH inhibitor therapy. Nat Commun, 2019. 10(1): p. 5829.
- Crowther, A.J., et al., Radiation Sensitivity in a Preclinical Mouse Model of Medulloblastoma Relies on the Function of the Intrinsic Apoptotic Pathway. Cancer Res, 2016. 76(11): p. 3211-23.
- Vanner, R.J., et al., Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell, 2014. 26(1): p. 33-47.
- Jessa, S., et al., Stalled developmental programs at the root of pediatric brain tumors. Nat Genet, 2019. 51(12): p. 1702-1713.
- Morrissy, A.S., et al., Divergent clonal selection dominates medulloblastoma at recurrence. Nature, 2016. 529(7586): p. 351-7.
- Xu, Z., et al., OLIG2 Is a Determinant for the Relapse of MYC-Amplified Medulloblastoma. Clin Cancer Res, 2022: p. OF1-OF14.
- Zhang, L., et al., Single-Cell Transcriptomics in Medulloblastoma Reveals Tumor-Initiating Progenitors and Oncogenic Cascades during Tumorigenesis and Relapse. Cancer Cell, 2019. 36(3): p. 302-318 e7.
- Desai, K., et al., OLIG2 mediates a rare targetable stem cell fate transition in sonic hedgehog medulloblastoma. Nature Communications, 2025. 16(1): p. 1092.
- Henley, M.J. and A.N. Koehler, Advances in targeting 'undruggable' transcription factors with small molecules. Nat Rev Drug Discov, 2021. 20(9): p. 669-688.
- Bushweller, J.H., Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer, 2019. 19(11): p. 611-624.
- Torres-Machorro, A.L., Homodimeric and Heterodimeric Interactions among Vertebrate Basic Helix-Loop-Helix Transcription Factors. Int J Mol Sci, 2021. 22(23).
- Edwards, A.L., et al., Challenges in Targeting a Basic Helix-Loop-Helix Transcription Factor with Hydrocarbon-Stapled Peptides. ACS Chem Biol, 2016. 11(11): p. 3146-3153.
- Tsigelny, I.F., et al., Multiple spatially related pharmacophores define small molecule inhibitors of OLIG2 in glioblastoma. Oncotarget, 2017. 8(14): p. 22370-22384.
- Alton, G. and S. Kesari, Novel small molecule inhibitors of the OLIG2 transcription factor: promising new therapeutics for glioblastoma. Future Oncol, 2016. 12(8): p. 1001-4.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in