Validation of the newly-revised International Staging System in relapsed/refractory multiple myeloma
Published in Cancer and General & Internal Medicine
December 17, 2024
The International Staging System (ISS) was developed as a simple, reliable, staging system for multiple myeloma to assist patient classification and stratification to facilitate prediction of disease progression risk. The ISS underwent its first revision (R-ISS) in 2015 to account for the prognostic importance of serum lactate dehydrogenase levels and certain high-risk cytogenetic abnormalities.1,2 However, there was still significant heterogeneity in patients classified as R-ISS Stage II.3 The presence of gain or amplification of 1q21 (1q21+) has been determined to be a significant predictor of both progression-free survival (PFS) and overall survival (OS) among patients with newly diagnosed multiple myeloma.4 Thus, the R-ISS has since been further revised (R2-ISS) to improve the ability to discriminate between patients classified as “intermediate risk” in the R-ISS by splitting the group into low-intermediate (R2-ISS Stage II) and intermediate-high (R2-ISS Stage III) via the introduction 1q21+ to the scoring schema. While the R2-ISS has been validated with data from clinical trials of patients with newly diagnosed multiple myeloma, its value as a prognostic scoring system has not been explored in patients with relapsed/refractory disease.
Isatuximab is a monoclonal antibody that targets a unique epitope of CD38, a transmembrane glycoprotein uniformly expressed on myeloma cells.5-7 Isatuximab kills myeloma cells via multiple mechanisms, including antibody-directed cellular cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, apoptosis, direct activation of natural killer cells, and inhibition of CD38 ectoenzyme activity.5-8 Isatuximab is approved for use in several countries to treat adults with relapsed/refractory multiple myeloma in combination with either pomalidomide and dexamethasone (Pd) or carfilzomib and dexamethasone (Kd).9-11 We have been studying the safety and efficacy of isatuximab in combination with standard-of-care treatment regimens in relapsed/refractory multiple myeloma. The primary aim of our analysis was to validate the prognostic value of the R2-ISS staging system in patients with relapsed or refractory multiple myeloma using datasets from the global Phase 3 ICARIA-MM and IKEMA trials, which investigated isatuximab in combination with Pd and Kd, respectively, in order to better predict disease progression risk after targeted therapy in this patient population.
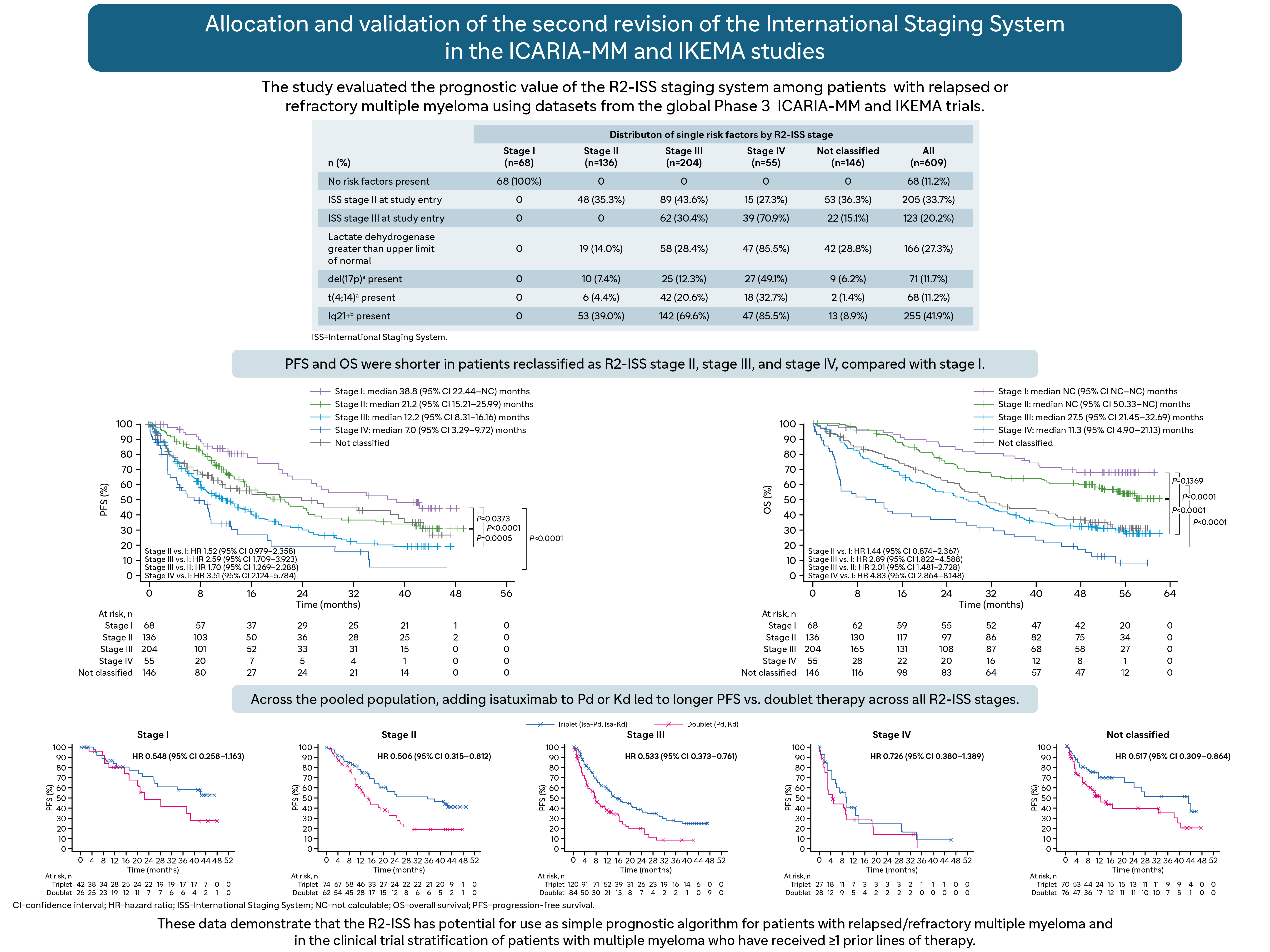
In this analysis, we pooled patients from the treatment and control arms of the ICARIA-MM (n=307)12,13 and IKEMA (n=302)14,15 trials for reallocation into R2-ISS stages using the revised scoring strategy. We assigned a score value (in brackets) to available individual prognostic risk factors considered for R2-ISS staging: ISS Stage II [1.0]; ISS Stage III [1.5]; lactate dehydrogenase above the upper limit of normal [1.0]; presence of del(17p) [1.0]; presence of t(4;14) [1.0]; and presence of 1q21+ [0.5]. We used the sum of risk factor values to classify patients according to R2-ISS stage, as follows: 0, Stage I; 0.5 to 1.0, Stage II; 1.5 to 2.5, Stage III, and ≥3.0, Stage IV. To minimize the number of patients deemed unclassifiable, we made an allowance for missing data when the sum of available risk factors reached a specific threshold. Early relapse was defined as relapsed <12 months from initiation of the most recent line of therapy for patients with ≥2 prior lines of therapy; relapsed <18 months for patients with 1 prior line of therapy; or relapsed <12 months from autologous stem cell transplantation.
Using this scoring strategy, we were able to classify more patients with early relapse as R2-ISS Stages III and IV (51% vs 42%) than R2-ISS Stages I and II (24% and 33%). In addition, more patients from ICARIA-MM (30.9%) were missing 1q21+ data compared with patients from IKEMA (11.9%). Of the 609 pooled patients, we reclassified 68 as R2-ISS Stage I, 136 as R2-ISS Stage II, 204 as R2-ISS Stage III, and 55 as R2-ISS Stage IV; 146 were not classified.
After a median follow-up duration of 11.6 months (ICARIA-MM) and 44 months (IKEMA), PFS was shorter in patients reclassified as R2-ISS Stage II, Stage III, and Stage IV, compared with Stage I. Consistent with R2-ISS, our data showed that median PFS decreased with increasing stage. After a median follow-up duration of 52.4 months (ICARIA-MM) and 56.6 months (IKEMA), OS was also shorter among patients reclassified as R2-ISS Stage II, Stage III, and Stage IV, compared with Stage I. Despite median OS not being reached for Stages I and II, we observed a clear separation of the curves. The presence of individual R2-ISS risk factors (compared with their absence) was similarly associated with shorter PFS and OS. Across the pooled population, adding isatuximab to Pd or Kd led to longer PFS vs doublet therapy across all R2-ISS stages; we observed similar findings for OS results.
To our knowledge, this is the first study to validate the R2-ISS in patients with relapsed/refractory multiple myeloma and in patients treated with a CD38 monoclonal antibody, using pooled data from two Phase 3 studies. In patients with relapsed or refractory multiple myeloma, R2-ISS improved discrimination of the large number of patients that the R-ISS classified as “intermediate-risk” by splitting this group into R2-ISS Stage II or III, as evidenced by a more even distribution of patients among the four R2-ISS stages. Additionally, we reported improved discrimination in terms of differences in PFS and OS between patients reclassified as R2-ISS Stages II and III, with significantly worse survival observed in Stage III patients.
Our findings also highlight the benefit of isatuximab-based triplet therapy vs immunomodulatory drug-based or proteasome inhibitor-based doublet therapy across patients with relapsed/refractory multiple myeloma, including those with early relapse. We also observed a benefit of isatuximab-based triplet therapy vs double therapy in patients with high-risk disease, as evidenced by the longer PFS in patients classified as R2-ISS Stage III or Stage IV. Our results echo findings from previous analyses that isatuximab use is advantageous in higher risk patients, such as those with extramedullary disease or high-risk cytogenetics.16-18
References
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23(15): 3412-20.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol 2015; 33(26): 2863-9.
- Schavgoulidze A, Lauwers-Cances V, Perrot A, et al. Heterogeneity in long-term outcomes for patients with Revised International Staging System stage II, newly diagnosed multiple myeloma. Haematologica 2023; 108(5): 1374-84.
- D'Agostino M, Cairns DA, Lahuerta JJ, et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J Clin Oncol 2022; 40(29): 3406-18.
- Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res 2014; 20(17): 4574-83.
- Martin TG, Corzo K, Chiron M, et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells 2019; 8(12).
- van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018; 131(1): 13-29.
- Zhu C, Song Z, Wang A, et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front Immunol 2020; 11: 1771.
- Sarclisa® (isatuximab-irfc). Bridgewater, NJ: Sanofi-Aventis U.S. LLC. 2024. https://products.sanofi.us/Sarclisa/sarclisa.pdf. Accessed October 22, 2024.
- European Medicines Agency (EMA). Medicines. Sarclisa. 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/sarclisa. Accessed October 22, 2024.
- Pharmaceuticals and Medical Devices Agency. Sarclisa. 2020. https://www.pmda.go.jp/files/000242148.pdf. Accessed October 22, 2024.
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet 2019; 394(10214): 2096-107.
- Richardson PG, Perrot A, San-Miguel J, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Lancet Oncol 2022; 23(3): 416-27.
- Martin T, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized Phase 3 study. Blood Cancer J 2023; 13(1): 72.
- Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet 2021; 397(10292): 2361-71.
- Beksac M, Spicka I, Hajek R, et al. Evaluation of isatuximab in patients with soft-tissue plasmacytomas: An analysis from ICARIA-MM and IKEMA. Leuk Res 2022; 122: 106948.
- Harrison SJ, Perrot A, Alegre A, et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br J Haematol 2021; 194(1): 120-31.
- Spicka I, Moreau P, Martin TG, et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high-risk cytogenetics: IKEMA subgroup analysis. Eur J Haematol 2022; 109(5): 504-12.
Follow the Topic
-
Blood Cancer Journal

This journal seeks to publish articles of the highest quality related to hematologic malignancies and related disorders.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in