Breakthrough in Stereoselective C(sp³)–H Alkylation: Nickel Catalysis Unlocks Efficient Synthesis of Chiral Heterocycles
Published in Chemistry
Background
The conversion of inexpensive and readily available aliphatic hydrocarbons into high-value chiral compounds via C(sp³)–H functionalization has attracted significant academic and industrial interest due to its inherent atom economy, step economy, and abundant starting materials. In recent years, the combination of hydrogen atom transfer (HAT) with transition metal-catalyzed cross-coupling has emerged as a powerful tool for enantioselective C(sp³)–H functionalization. Current strategies based on HAT/transition metal catalysis, such as C(sp³)–H cyanation, arylation, alkenylation, alkynylation, and acylation, have been successfully implemented. However, enantioselective C(sp³)–H alkylation of saturated hydrocarbons lacking directing groups remains highly challenging.
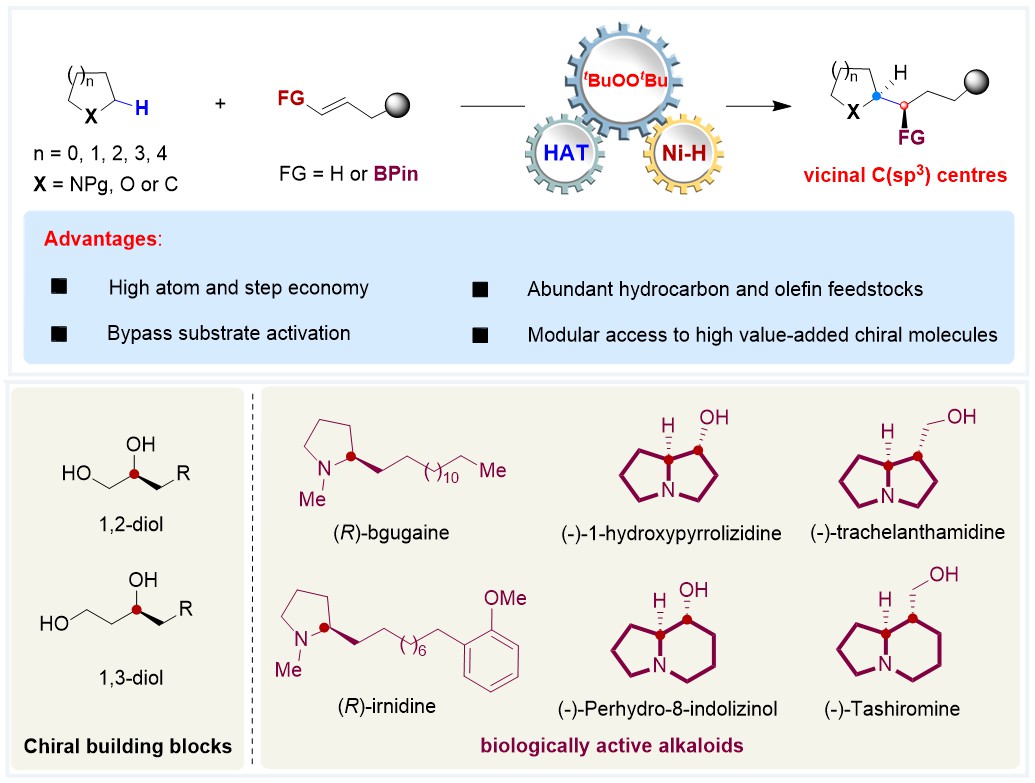
Research Progress by Kong Group
Our research team has been dedicated to achieving C(sp³)–H functionalization of various saturated oxygen heterocycles. In 2023, we successfully realized enantioselective C(sp³)–H arylation and alkenylation of oxacycles via a photoinduced HAT/nickel dual catalysis strategy (J. Am. Chem. Soc. 2023, 145, 5231–5241). In this work, C(sp³)–H bonds on oxygen heterocycles with multiple stereocenters were efficiently transformed into stereoselective C(sp³)–C(sp²) bonds. Building on this foundation, we further activated the C(sp³)–H bonds of 1-deoxyglycosides using a photoinduced HAT strategy, achieving regioselective and stereoselective functionalization under the control of a nickel catalyst and chiral ligands (Nat. Chem. 2024, 16, 2054–2065). After accomplishing stereoselective functionalization of various saturated oxygen heterocycles, we tackled the more challenging enantioselective C(sp³)–H alkylation of undirected saturated nitrogen/oxygen heterocycles—a reaction previously unreported. By Inspiring on nickel-catalysed peroxide-mediated C(sp3)–H functionalization and NiH-catalysed olefin hydroalkylation systems, we developed a stereoselective C(sp³)–H alkylation of saturated nitrogen/oxygen heterocycles using olefins.
Key Features of the Method
The optimized reaction system combines nickel bromide with chiral bis(oxazoline) ligands, silanes as hydride sources, and peroxides as hydrogen atom transfer (HAT) reagents. Key highlights include:
- High Enantioselectivity: Products are obtained with up to 99% enantiomeric excess (e.e.), making this method highly attractive for asymmetric synthesis.
- Functional Group Tolerance: Sensitive functional groups, including esters, ethers, halides, and boronate esters, remain intact during the reaction, enabling late-stage functionalization of complex molecules.
- Broad Substrate Scope: The reaction is compatible with a wide range of saturated nitrogen- and oxygen-containing heterocycles, such as pyrrolidines, piperidines, tetrahydrofuran, acetonide-protected ethylene glycol and 1,3-propanediol, as well as diverse olefins (terminal, internal, and cyclic).
- Diastereoselective Control: The enantio- and diastereoselective C(sp3)–H alkylation of saturated hydrocarbons with alkenyl boronates has been achieved, enabling the synthesis of versatile alkyl boronates containing 1,2-adjacent C(sp3) stereocentres.
- Synthesis of Bioactive Alkaloids: Streamlined routes to natural products like (−)-coniceine, (R)-bgugaine, and (−)-trachelanthamidine highlight the method’s efficiency in reducing synthetic steps compared to traditional approaches.
Conclusion
This study represents a significant leap forward in asymmetric catalysis, merging radical chemistry with nickel catalysis to unlock previously inaccessible chemical space. By offering a green, scalable, and stereoselective platform for C(sp³)–H alkylation, the work bridges the gap between academic innovation and industrial application.
For further details, read the full article in Nature Chemistry:
Direct Stereoselective C(sp³)–H Alkylation of Saturated Heterocycles Using Olefins
DOI: 10.1038/s41557-025-01747-6
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in