Extreme wrinkling of the nuclear lamina is a morphological marker of cancer
Published in Bioengineering & Biotechnology, Cancer, and Computational Sciences
Two questions
Nuclear atypia is a defining, diagnostically-useful feature of many cancers. Nuclear atypia includes features like irregular nuclear shape, size, and heterochromatin distribution, among other features1. Nuclear atypia is generally qualitatively determined by visual examination of hematoxylin and eosin (H&E) stained tissue samples. This qualitative approach inevitably introduces inter- and intra-pathologist variability in assessments of nuclear atypia. As such, there is a need for quantifiable and unbiased measurements of nuclear atypia.
The cause of nuclear atypia in cancer is not fully understood. Studies of nuclear mechanics have shown that the nuclear lamina, a structure which underlies the nuclear envelope, helps determine the shape of the nucleus. These studies are usually performed in 2D cell culture, where the nuclear lamina is smoothed out because the nucleus is flattened by cell spreading. When cells are prevented from spreading, the lamina takes on a wrinkled appearance. Given that cells are less spread in vivo than on the flat surfaces of 2D culture, the nuclear lamina in vivo may be wrinkled rather than smooth. This is a feature of the recent nuclear “drop model” proposed by the Lele and Dickinson labs. The model posits that the wrinkles that appear when a nucleus is rounded—i.e. when its shape approaches a sphere—are a manifestation of the “excess surface area” of the lamina over the area of a sphere of the nucleus’s volume2. This excess laminar area, which develops post-mitosis around irregularly packed chromosomes3, allows nuclei to assume diverse shapes with little mechanical resistance4. The nucleus becomes ‘stiff’ to shape changes only when it is flattened and the lamina is smooth and taut, whereupon it resists extension5.
In this work, we sought to test the above aspects of the drop model in patient tissues. We combined our interests in nuclear mechanobiology and pathology in this paper, to answer these questions: (1) Are most nuclei in patient tissues wrinkled, and do these wrinkles facilitate diverse nuclear shapes as predicted by the drop model? and (2) Is nuclear laminar wrinkling a quantifiable morphological marker of cancer?
Our findings
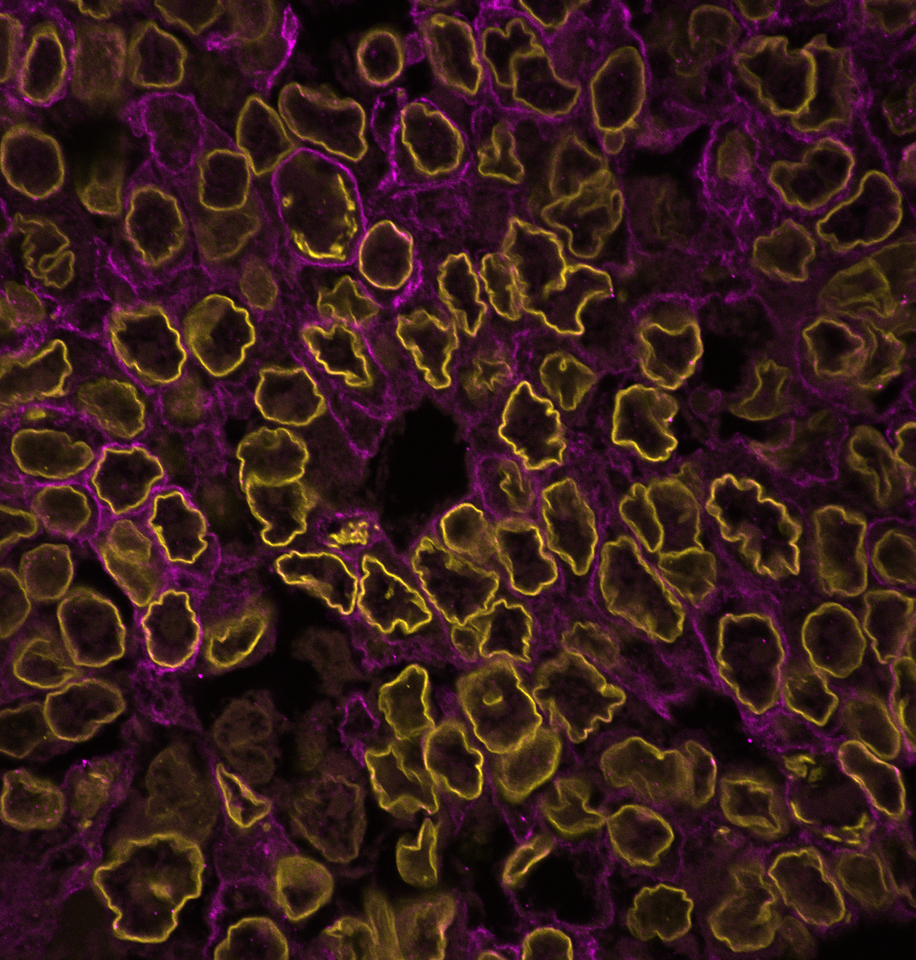
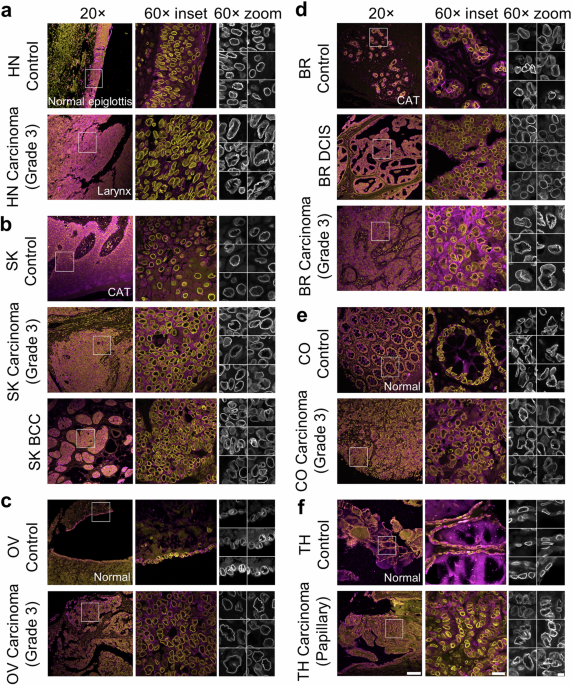
To answer these questions, we immunostained patient tissues for the nuclear lamina and imaged them at a high resolution. Nuclear laminar wrinkles were present in both control and cancer tissue, from head and neck, skin, ovarian, breast, colon, and thyroid tissues (see Figure 1 for examples of nuclear wrinkling). Nuclear wrinkling was not visible in H&E-stained tissues.
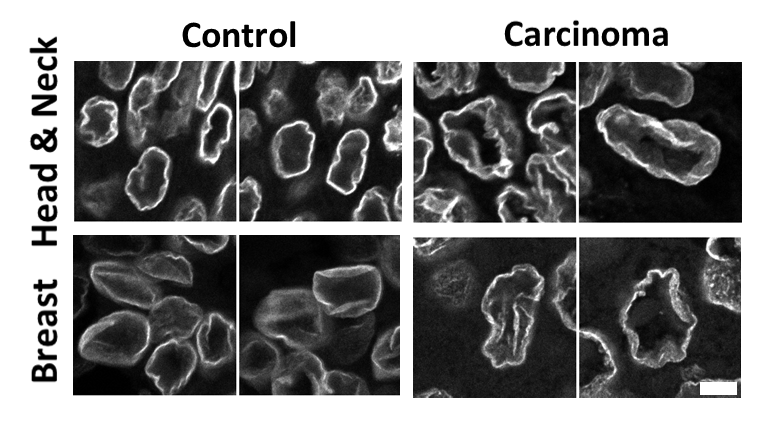
Figure 1: Nuclear wrinkling in patient tissues immunostained for Lamin B1 and imaged at 60X. Scale bar is 5 µm.
There was an exception in pure breast ductal carcinoma in situ (DCIS), which had many smooth nuclei, unlike other tissue types. We used this difference to assess the second part of our first question: do nuclear wrinkles allow for diverse nuclear shapes? We compared nuclei from DCIS to the mostly wrinkled nuclei from invasive breast carcinoma and found that nuclear shapes were indeed more diverse in invasive breast carcinoma.
The near-ubiquitous nature of nuclear wrinkles required testing to determine whether they might be tissue processing artifacts. We used formalin-fixed, paraffin-embedded (FFPE) tissue for this study. This type of tissue is widespread in pathology, but it requires intense processing steps for immunostaining: xylene and ethanol washes, rehydration, and antigen retrieval (a heat and pH-based method to reduce extensive crosslinking from 24-48 hour-long formalin fixation). Therefore, we also immunostained frozen patient tissue for the nuclear lamina to look for nuclear wrinkles, as it has different, and fewer, processing steps. Nuclear wrinkles were present in this tissue also. Additionally, we performed some internal controls on FFPE tissue.
Once we were satisfied that nuclear wrinkles in control and cancer tissue were not artifacts of tissue processing, we asked if the extent and/or type of wrinkling was altered in cancer, and could be leveraged as a morphological marker. To test this, we visually classified nuclei based on their wrinkling into four classes: smooth nuclei without wrinkles, nuclei with low frequency contour variations, nuclei with high frequency contour variations, and nuclei with inner wrinkles (see Figure 2 for examples of nuclei in these categories). We considered the last two classes to both be examples of extreme nuclear wrinkling.
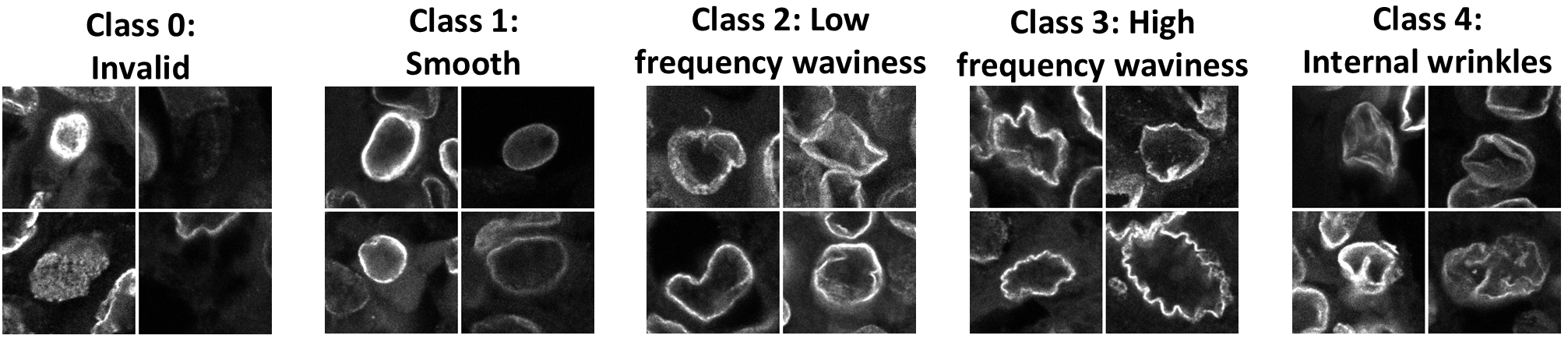
To avoid the bias that is inherent in qualitative assessments of nuclear wrinkling, we used transfer learning to employ a pre-trained ResNet50 deep learning model6 to classify nuclei from patient samples. We cropped individual nuclei from our images of patient tissues and restricted analysis to nuclei from pan-cytokeratin-expressing cells, so only carcinoma and control epithelial cells were considered.
Running thousands of images of nuclei through this model revealed that low frequency contour waviness was the most common wrinkling type in each tissue, reinforcing our finding that most tissues had wrinkled nuclei. Furthermore, our analysis demonstrated that tissues from cancer patients tended to have more extremely wrinkled nuclei than control tissues. Additionally, the amount of extremely wrinkled nuclei tended to be higher in cases with higher cancer grade and lymph node involvement.
To verify our deep learning results, we performed Fourier analysis of nuclear contours7. We adapted a custom segmentation method8 to capture micron- and submicron-variations in the nuclear contour for this purpose, as the more common segmentation methods smoothed out the high frequency contour variations clearly visible in the nuclear lamina. This analysis confirmed our deep learning findings—the nuclear contour was more irregular in cancer tissues compared to control tissues.
We tested three possible causes of extreme nuclear wrinkling: a loss of lamin A/C in cancer9, the presence of perinuclear actin fibers10, and location in cancer cell clusters: but none of these phenomenon satisfactorily explained the prevalence of extreme nuclear wrinkling. It is possible that cell rounding occurs in cancer tissues owing to a general loss of tissue structure which promotes more rounded and hence wrinkled nuclei11, but testing this hypothesis requires 3D imaging of cells in thicker tissue. This work is currently underway in the lab.
Overall, our data indicate that nuclear wrinkles are a feature of human tissue—both normal and cancer—consistent with the nuclear drop model. Yet, extreme nuclear laminar wrinkling is a morphological marker of cancer tissue. The detection of laminar wrinkling with AI approaches could improve the accuracy of cancer diagnosis and prognosis.
Read more here: Wang, TC., Dollahon, C.R., Mishra, S. et al. Extreme wrinkling of the nuclear lamina is a morphological marker of cancer. npj Precis. Onc. 8, 276 (2024). https://doi.org/10.1038/s41698-024-00775-8
Multiple contributors: Tanmay P. Lele also contributed to this post.
References:
1. Fischer, E. G. Nuclear morphology and the biology of cancer cells. Acta Cytol. 64, 511–519 (2020).
2. Dickinson, R. B. & Lele, T. P. Nuclear shapes are geometrically determined by the excess surface area of the nuclear lamina. Front Cell Dev. Biol. 11, 1058727 (2023).
3. Naetar, N., Ferraioli, S. & Foisner, R. Lamins in the nuclear interior - life outside the lamina. J. Cell Sci. 130, 2087–2096 (2017).
4. Dickinson, R. B. & Lele, T. P. A new function for nuclear lamins: providing surface tension to the nuclear drop. Curr. Opin. Biomed. Eng. 28, https://doi.org/10.1016/j.cobme.2023.100483 (2023).
5. Tang, W. et al. Indentation induces instantaneous nuclear stiffening and unfolding of nuclear envelope wrinkles. Proc. Natl Acad. Sci. USA 120, e2307356120 (2023).
6. He, K. M., Zhang, X. Y., Ren, S. Q. & Sun, J. Deep Residual Learning for Image Recognition. Proc Cvpr Ieee, 770-778, https://doi.org/10.1109/Cvpr.2016.90 (2016).
7. Tamashunas, A. C. et al. High-throughput gene screen reveals modulators of nuclear shape. Mol. Biol. cell 31, 1392–1402 (2020).
8. Wu, J. et al. Effects of dynein on microtubule mechanics and centrosome positioning. Mol. Biol. Cell 22, 4834–4841 (2011).
9. Irianto, J., Pfeifer, C. R., Ivanovska, I. L., Swift, J. & Discher, D. E. Nuclear lamins in cancer. Cell Mol. Bioeng. 9, 258–267 (2016).
10. Cosgrove, B. D. et al. Nuclear envelope wrinkling predicts mesenchymal progenitor cell mechano-response in 2D and 3D microenvironments. Biomaterials 270, 120662 (2021).
11. Lele, T. P., Dickinson, R. B. & Gundersen, G. G. Mechanical principles of nuclear shaping and positioning. J. Cell Biol. 217, 3330–3342 (2018).
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Good job