How could innate immunity pass unnoticed for so long ?
Published in Biomedical Research
Presenting tissue/microenvironment control as an embodiment of how to intervene in the constitutive cell-autonomous response targeting an optimal interactome for inducement has been daunting. It defies pharmacology as it is presently conceived and requires new multivalent mechanistic restatements with a pristine lexicon necessarily unfamiliar to most.
Discoveries of resistance in innate immunity have been described in separate chapters and have never been explained comprehensively. "The cell's autonomous response window for advanced healing" is a first attempt to collate such knowledge and explain how it functions collectively, well before the response summons the late inflammatory components of resistance (adaptive immunity). Such a view is based on solid basic science and strong clinical evidence, but in some aspects of the constitutive mechanistic construct, it lacks validation in cell and molecular research. With further development, elucidation at such a level will hopefully be consistent with the theorized molecular aspect.
The lines of evidence that strongly motivated writing the article on the innate immunity construct consisted of the ideal regenerative-healing outcome observed in early research and translated to the clinic, and the formidable defense against infection noticed in injured tissues, both quite obvious and unprecedented. The first line of evidence is described in detail in the article. But new evidence on innate immunity antimicrobial defense, which is not included in the article, is referenced and briefly discussed below, adding robust validation to extensive findings of injured tissue combating and remaining free of infection.
For decades, it was believed that the primary function of peptides derived from proteasome degradation was antigen presentation by the major histocompatibility complex class I to launch adaptive T-cell immunity. But research during the last decade has clarified that the function extends to the generation of antimicrobial peptides with cationic properties on an unimaginable scale and variety, through further tryptic proteosomal degradation caused by changes in proteomic composition and function and induced by pathogens to eventually induce the extraction of these cationic fractions, which perforate the pathogen's membrane, causing the arrest of bacterial growth; without adaptive participation, and still within the first line of defense (2) .
In 2011, Ponpuak and Deretic (3) described that in autophagy, phagosomes are more than just a residence vacuole for M. tuberculosis. The adaptor molecule p62, or sequestosome 1, interacts with phagosomal substrates and sequesters harmless cytosolic components (the ribosomal protein rpS30, the precursor FAU, and ubiquitin) for degradation, releasing antimicrobial peptides from used or discarded material. This gives the phagosome lysosomal and maturation characteristics, which, along with the capture of hydrolases and the intermediate mobilization of substrates and their acidification, eventually lead to its fusion into a true autolysosome. The assumption that the outcome of this transformation was to provide simply free amino acids to be recycled during periods of starvation is thus challenged. It has since been elucidated that the proteolysis of these cytosolic proteins ends in a mixture of cryptic peptides with antimicrobial power, constituting the new natural antibiotics called "cryptides," which are generated from discarded biological material. Or by specific chain formations from bacterial surface proteins carrying the ubiquitin tag, which may be plucked from the membrane and fed to the proteasomes, seriously compromising the integrity of the bacterial cell wall and the spillage of pathogen contents in the cytosol(5). In short, the transformation and recycling enhance a valuable post-function, such as eliminating mycobacteria, notorious for their formidable resistance to disposal; see Fig. 1.
Fig. 1
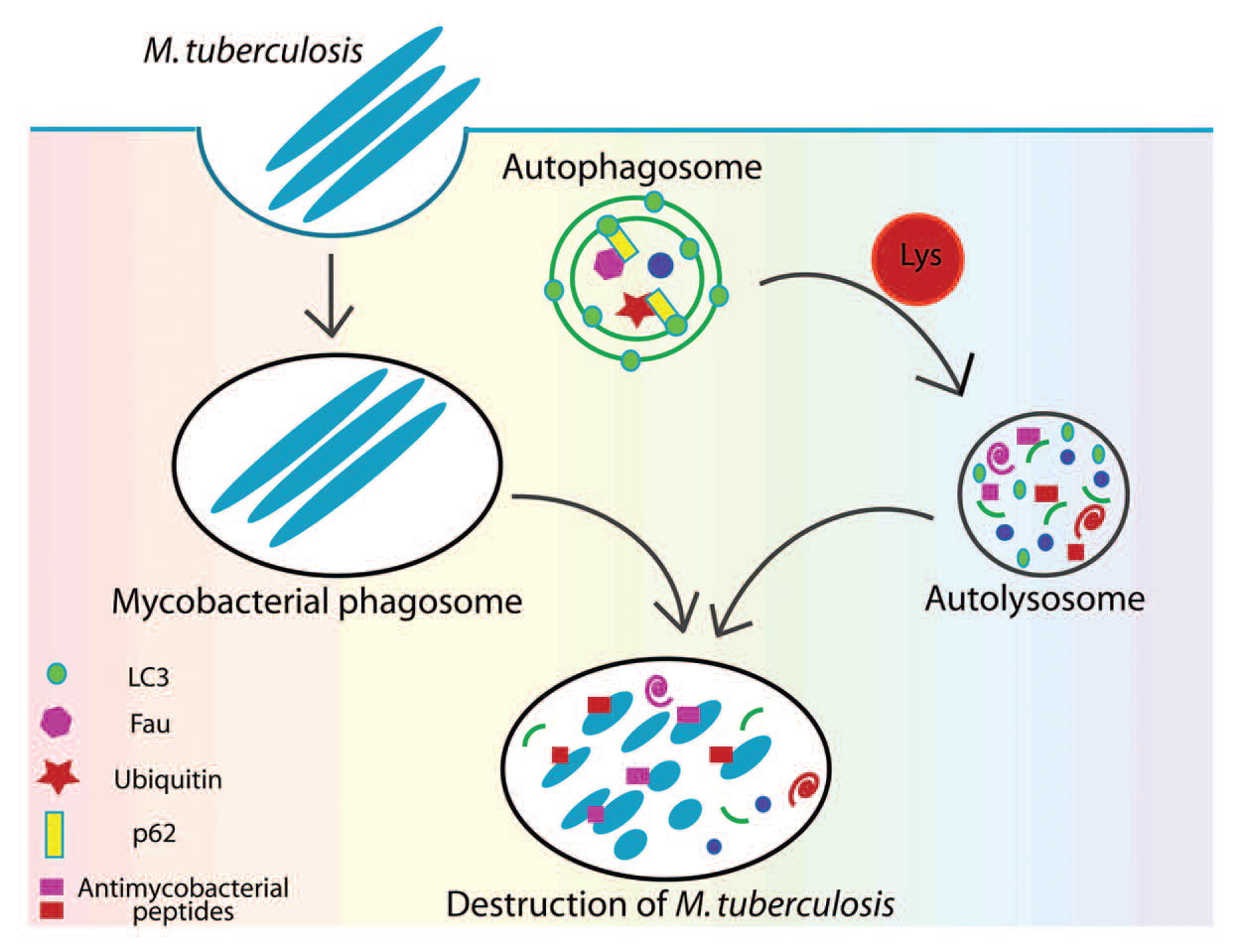 Elimination of M. tuberculosis via autophagy and p62. Mycobacterium is phagocytosed by macrophages and resides within the phagosome for at least some time. When autophagy is induced, p62, as a bifunctional agent, interacts with autophagic substrates and LC3, sequestering additionally cytosolic substrates. Autophagosome maturation and hydrolase capture induce the degradation of all substrates and their conversion into peptides (cryptides) with antimicrobial activity. Taken from (3)
Elimination of M. tuberculosis via autophagy and p62. Mycobacterium is phagocytosed by macrophages and resides within the phagosome for at least some time. When autophagy is induced, p62, as a bifunctional agent, interacts with autophagic substrates and LC3, sequestering additionally cytosolic substrates. Autophagosome maturation and hydrolase capture induce the degradation of all substrates and their conversion into peptides (cryptides) with antimicrobial activity. Taken from (3)
Fig. 2
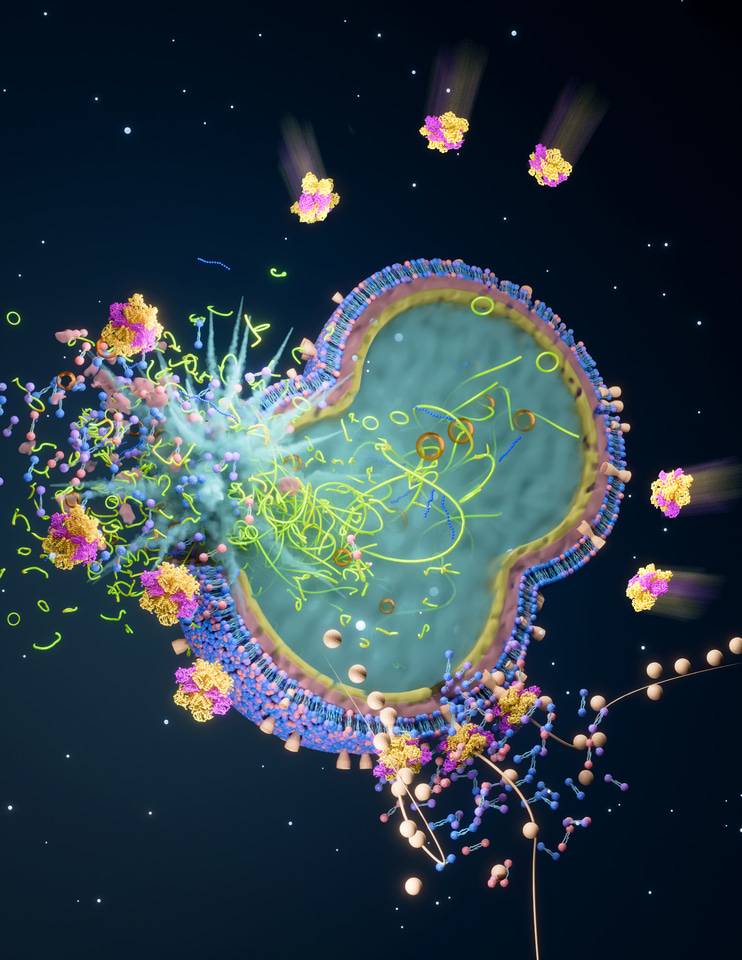 Bacterial plucking, taken from (5)
Bacterial plucking, taken from (5)
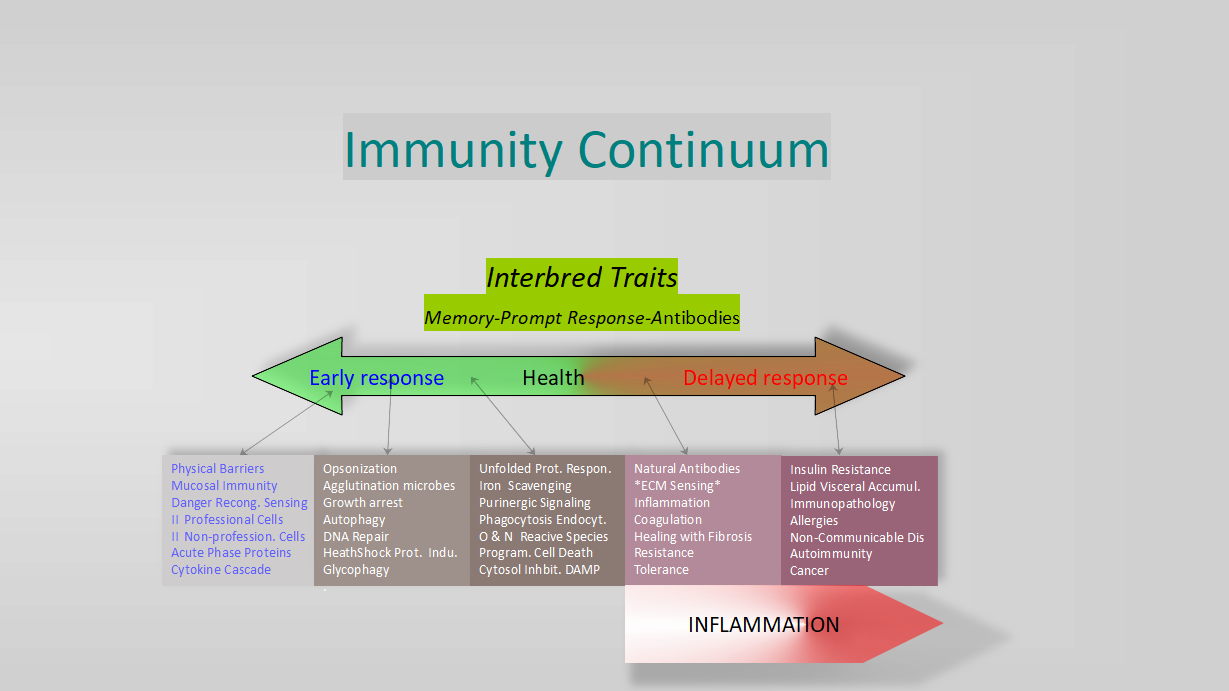
In March 2025, Goldberg et al. (2) clarified that the production of cryptides with cationic properties also occurs in proteasomal function, which for decades was thought to be limited to generating the histocompatibility complex I or degrading inhibitory proteins (IκBs) to activate nuclear factor kappa B (NF-κB) or to activate protein precursors with transcriptional regulatory functions, such as Spt23p, Mga2p, and Epe1. However, they also showed that the source of extraction is within substrates extracted from the cytosol, as in autophagy. In addition to being a first-order evolutionary step, antibacterial peptides are found in a wide variety of tissues and cells and exert broad-spectrum activity against bacteria, fungi, viruses, and some parasites. In addition to their cationic action, they modulate the transcriptional and translational activity in the host. These peptides have been termed proteasome-derived peptides (PSEI PDDPs) by Goldberg et al. They are constitutively produced, but their production can also be induced as a response to infection. A proteasome regulatory unit, PSME3, promotes tryptic degradation, increasing peptides with cationic terminals. Computational analyses of this phenomenon show that most proteasome proteins are prone to this degradation, and therefore, the proteasome's potential for PDDP production is quite powerful. An in silico identification using the Pepsickle algorithm (4) of potential cleavage sites to obtain amino acids between 10 and 50 units in length, rated with a minimum efficacy range based on structural comparison to peptides with proven antibacterial activity, identified 270,872 putative peptides with cationic carboxyl terminals. In other words, 92% of the annotated genes contain at least one peptide with a cationic terminal. This illustrates the enormous potential of inducing autonomous cellular immunity to treat tissue injuries, without the risk of infection, and with a strong likelihood of generating a tailored antimicrobial to combat the pathogens involved in each specific case. Another interbred function to be added to the Immunity Continuum, Fig. 1 of the article, shown as a poster in this post, introduces the concept of early and accurate immune specificity through multitudinous offerings. Taken together, new thinking is necessary to improve resistance to infection and immunity, seen as a continuum, to finally overcome it.
The identification of Cubrisoft's (the intervention proposed in the article) mechanism of action within the autonomous cellular immunity and the regular absence of infection in lesions treated with this product are consistent with the research described by Goldberg et al. and Sourav et al.. Resource-sparing is at the heart of early resistance, which profits from cytosolic protein waste and a low energetic expenditure, in contrast to the reckless late adaptive defense response(1). This evidence places Cubrisoft at the forefront of combating infection in injured tissues without toxic or unwanted effects, creating optimal conditions for the regenerative repair described in "The cell's autonomous response window for advanced healing" (1).
It is not only resistance but switching into tolerance also, to preserve tissue health and survive
As proposed in the article, healing with fibrosis is a second-order deal, not really appreciated until cell reprogramming became known. Mounting research since the discovery has unveiled multiple ways within the innate immunity through which inflammation is avoided, resulting in regeneration and no fibrosis. And just at the very limit, several options can still be found for circumventing a microenvironment with a rigid ECM and the consequent immune cell escape.
Nor is it still tenable that the immune escape gets rid of the pathogen and clears the infection, since the clinical findings of the article and the vast opportunities that AMPs offer for keeping injuries clean are now a fact but additionally for the refinements recently discovered that natural killer (NK) and natural killer like cells (NK-like) perform, in both pathogen clearance and maintenance of tissue health. That is, a major line of circulating/tissue-resident lymphocytes ensures a resistance/tolerance role before giving way to adaptive immunity and avoids tissue damage with a striking resemblance to how intruders get torn apart by AMPs, but together with amphiregulin production. Not only that, but vast opportunities have also been identified regarding the reprogramming of lung tissue for restoration to health, without hindrance to gas exchange, as a preferable outcome, which are all amenable if tissue/microenvironment control is sought (the foremost way of communicating with the interactome). (6) (7) (8)
It can now be posited that tissue damage control relies on several cellular and systemic responses that protect host parenchyma tissues from stress, dysfunction and/or damage to limit host disease severity without interfering with pathogen load. These are mostly metabolic adaptations and antioxidant redox responses that modulate tissue damage severity and, in essence, participate in maintaining tissue health. (9)
In other words, the description of the article of healing within the autonomous window needs further clarification of how tissue architecture is restored when used immediately after injury. However, it is a theme for a future article. Please consider this closing section of the commentary as an insight into the balance required to fight injury and what is to come in the article, which oscillates between resistance and tolerance to achieve ideal healing (future wide clinical standard practice) with cell reprogramming or paligenosis, and exquisitely avoiding tissue damage in the process of healing. Please also note that such innovative mechanisms are all principally within the innate immunity, and it is surprising how all this has passed unnoticed for so long.
References
1. Feoli -Tufi E, Redondo GM, Flores JS, Jara DP. The cell's autonomous response window for advanced healing. Discov Med [Internet]. October 10, 2025 [cited November 9, 2025];2(1):1-42. Available from: https://link.springer.com/article/10.1007/s44337-025-00478-4
2. Goldberg K, Lobov A, Antonello P, Shmueli MD, Yakir I, Weizman T, et al. Cell-autonomous innate immunity by proteasome-derived defense peptides. Nature [Internet]. Mar 2025 [cited 2025 Nov 9];639(8056):1032-41. Available at: https://www.nature.com/articles/s41586-025-08615-w
3. Ponpuak M, Deretic V. Autophagy and p62/sequestosome 1 generate neo-antimicrobial peptides (cryptides) from cytosolic proteins. Autophagy [Internet]. March 1, 2011 [cited November 9, 2025];7(3):336-7. Available from: https://doi.org/10.4161/auto.7.3.14500
4. Weeder BR, Wood MA, Li E, Nellore A, Thompson RF. Pepsickle rapidly and accurately predicts proteasomal cleavage sites for improved neoantigen identification. Bioinformatics [Internet]. Sep 2021;37(21):3723-33. Available at: https://doi.org/10.1093/bioinformatics/btab628
5. Ghosh Sourav, Roy Suvapriya, Baid Navin. Host AAA-ATPase VCP/p97 lyses ubiquitinated intracellular bacteria as an innate antimicrobial defence. Nature Microbiology 2025; 10(5):1099–1114 Available at: https://www.nature.com/articles/s41564-025-01984-y?utm_campaign=related_content&utm_source=HEALTH&utm_medium=communities
6. Schuster, I. S., Sng, X. Y. X., & Lau, C. M. (2023). Infection induces tissue-resident memory NK cells that safeguard tissue health. Immunity, 56(3), 531–546.
7. Vick, S. C., Domenjo-Vila, E., & Frutoso, M. (2025). Mucosal tissue NK cells tune their function between optimal anti-pathogen activity and tissue protection. bioRxiv.
8. Basil, M. C., Alysandratos, K.-D., & Kotton, D. N. (2024). Lung repair and regeneration: Advanced models and insights into human disease. Cell Stem Cell, 31(4), 439–454.
9. Soares, M. P., Gozzelino, R., & Weis, S. (2014). Tissue damage control in disease tolerance. Trends in Immunology, 35(10), 483–494.
Follow the Topic
-
Discover Medicine

This is a fully open access, peer-reviewed journal that supports multidisciplinary research and policy developments across the fields of medical and clinical science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Gender and Ethnic Disparities in Cardiovascular Health and Diseases
The global burden of cardiovascular disease (CVD) is alarming, with profound gender and ethnic disparities. Women are more likely to be underdiagnosed and undertreated, while ethnic minorities, including African Americans, Hispanics/Latinos, American Indians, and those in underserved communities, bear a disproportionate burden of CVD. Socioeconomic factors, inadequate healthcare access, and cultural barriers exacerbate these disparities. These disparities are not only a reflection of biological differences but also encompass a complex interplay of social determinants, healthcare access, and cultural factors. Understanding these dimensions is crucial for developing effective prevention and intervention strategies aimed at mitigating cardiovascular health inequalities. To bridge these gaps, rigorous research, robust data collection, and effective dissemination of findings are imperative in identifying these drivers.
This Collection will provide a platform for experts to share their research, insights, and solutions to address these pressing issues. We believe this Collection will contribute significantly to the ongoing conversation on health disparities and inform strategies to reduce the burden of CVD. We invite researchers and scientists to submit their original work to our Collection focused on gender and ethnic disparities in cardiovascular health and diseases. This Collection will contribute significantly to our understanding of the complex relationships between gender, ethnicity, and cardiovascular health outcomes and provide valuable insights into the drivers of these disparities and inform strategies for improvement.
Topics of interest include, but are not limited to:
- Gender differences in cardiovascular disease prevalence
- Ethnic disparities in treatment outcomes
- Sociocultural influences on cardiovascular health
- Impact of social determinants on heart disease risk
- Community-based interventions for at-risk populations
Keywords: Cardiovascular Disease Disparities, Gender and Ethnic Disparities in Health, Healthcare Access and Outcomes, Cardiovascular Health Equity, Racial and Ethnic Disparities in Cardiovascular Disease
This Collection supports and amplifies research related to SDG 3.
Publishing Model: Open Access
Deadline: Apr 14, 2026
Innovative Laboratory and Epidemiological Strategies to Reduce Mortality of Non-Communicable Chronic Diseases
Non-communicable chronic diseases (NCDs) and related conditions remain the leading causes of morbidity and mortality worldwide. These include two major categories: non-communicable chronic diseases—such as cardiovascular diseases, chronic respiratory diseases, and diabetes mellitus—and external causes, including accidents and violence. In parallel, advances in laboratory sciences and the growing field of precision medicine have opened new possibilities for early diagnosis, risk stratification, and personalized management. These approaches aim not only to identify individuals at greater risk of developing NCDs, but also to monitor disease progression and the emergence of complications or comorbidities. In this context, innovative laboratory methods and novel biomarkers are urgently needed to enhance disease detection and surveillance strategies. We therefore invite researchers to submit original articles, reviews, and case studies addressing the role of precision medicine in improving the surveillance and clinical management of chronic non-communicable diseases and their progression.
Keywords: Non-communicable chronic diseases; disease aggravation; biomarkers; laboratory methods; epidemiological aspects; individual risk classification; precision medicine; health promotion; surveillance; innovative laboratory approaches.
Publishing Model: Open Access
Deadline: Mar 23, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in