Tb bug can be bugged even by small frivolous signals
Published in Microbiology

Two component Signaling systems (TCSs) consists of a pair of proteins, the sensing protein is sensor histidine kinase (HK) and the response generating protein is response regulator (RR). TCSs are attractive drug targets known to cripple the pathogen’s adaptive responses to host immunity and drug regimens. We, at the Center of BioSystems Science and Engineering (BSSE) at Indian Institute of Science have recently unraveled a mechanism inherent to TCSs which allows the bug to ignore small frivolous signals thereby avoiding efforts to mount unnecessary and disproportionately large adaptive responses.
https://www.nature.com/articles/s41467-023-40095-2
Sequestration of HK by other non cognate RRs sets a signal detection threshold below which all incoming signals for the HK are ignored and diffused. Tb bug uses this simple design principle to define the extent of signal to ignore and respond to. Targeting such a sequestration interaction can render the bug to get bugged with even small stress signals posed by host immunity or drug regimens. Exploiting this finding to pin a sequestration interaction, in principle, can exhaust the pathogen enough to succumb to immune pressures. Pinning down protein complexes using molecular scaffolds have been an old trick of trade employed by pharma companies for antimicrobial drug discovery. This study informs them for potential sequestration interactions to exploit against the bug.
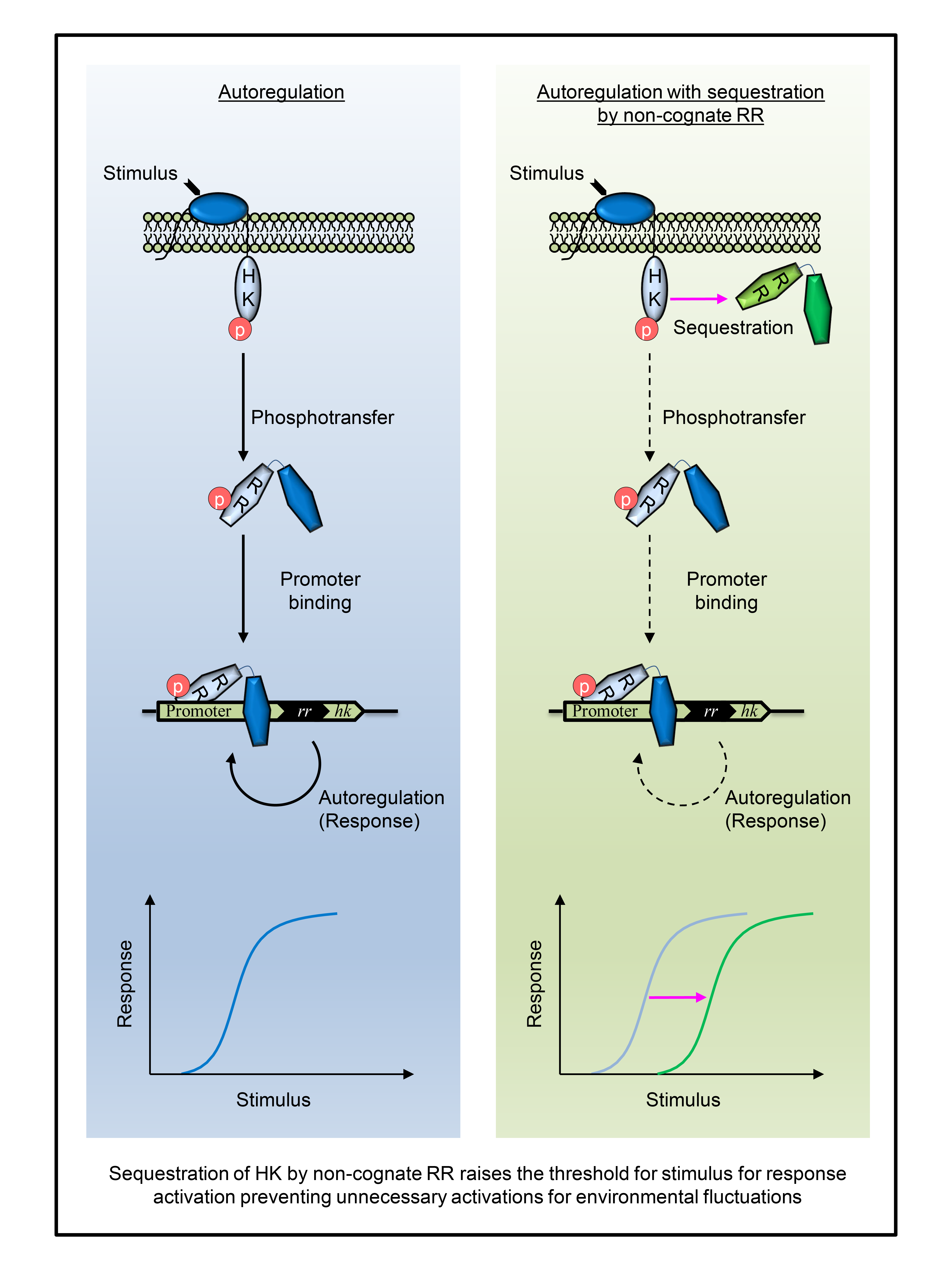
Specificity in TCSs, where HK interacts to its cognate RR to pass the upstream signal to generate adaptive response, is a norm. This ensures signal insulation and intuitively favors energy optimization for the microbes. There were a few studies which provided indirect evidence and hints for prevalence of non-specific interactions of HKs to non cognate RRs. Our finding that, most of mycobacterial HKs have higher affinities for non cognate RRs than their cognate counterparts, was an absolute surprise. This finding was validated by the classical technique- Isothermal Titration Calorimetry and counter validated by Surface Plasmon Resonance. Further, we showed effect of sequestration on cognate signaling in-vitro using kinetics, fitting it to a mathematical model yielded kinetic parameters which were subsequently employed in the model encompassing all signaling events to compute and visualize input-output attributes of sequestration. Finally, we also demonstrated sequestration in-vivo by probing its impact on downstream gene expression governed by the TCS in mycobacteria. The Covid-19 pandemic brought the world to a grinding halt and the experimental world for a longer time. The supply chain issues of key reagents caused severe delays in our experiments. This got further exasperated due to the in-vivo experiments involving mycobacteria which demanded Bio Safety Level (BSL) facilities. The BSL facilities across institutions were shutdown or repurposed for urgent covid-19 testing. This derailed our ability to perform crucial in-vivo experiments which was back on track when the third pandemic wave subsided. We would immensely thank the accommodative editor and their patience allowing longer revision timespan.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in