Uncovering the activation mechanism of a ligand-gated ion channel
Published in Neuroscience and Biomedical Research
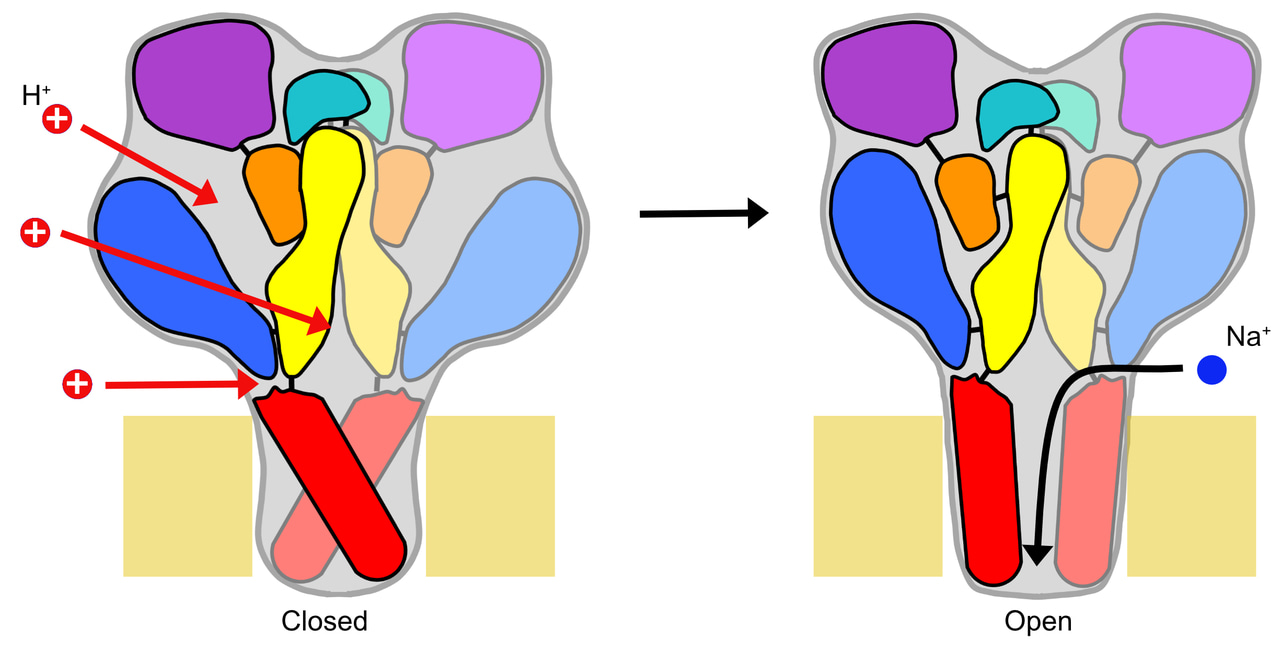
ASICs are low pH-activated Na channels of the nervous system, involved in the expression of fear, learning, pain sensation and neurodegeneration. Protons, the smallest ligands, can in principle bind to almost any amino acid residue that can be titrated, thus to Aspartate (Asp), Glutamate (Glu), Histidine (His) and Lysine (Lys). The ASIC1a trimeric channel contains 250 extracellular solvent-accessible titratable residues (84 per subunit). Previous studies suggested that protonation at several sites is required for activating the channels. Many studies have already identified residues whose mutation affects the ASIC pH dependence. It has however not been clear which residues are protonated when the pH is lowered, and which of them promote ASIC activation.
How to find the proton binding sites that are really involved in channel activation? We reasoned that those titratable residues are most likely involved in activation, whose protonation status changes upon acidification. This would alter the charge of the side chain, potentially inducing conformational changes that could then lead to channel opening. ASICs are activated by acidification of the extracellular solution from the physiological pH 7.4 to more acidic values, in the range of about pH6.5-5. The pKa defines the pH at which 50% of an acid or base exists in the protonated, and 50% in the deprotonated form. The ratio of the protonated / non-protonated form is most sensitive to pH changes if these occur in a pH range close to the pKa value. In the context of ASIC activation, residues would therefore change their protonation status most easily, if their pKa value is close to the range of the pH change.
Textbooks indicate pKa values of the different titratable side chains for isolated residues, i.e. without interactions with other amino acid residues. In a protein, these pKa values can be different from the textbook values, because they depend on electrostatic interactions between a given residue and its environment. Glu has a textbook pKa of ~4.2. Imagine there is either one isolated Glu residue, or there are two Glu residues close to each other. For the isolated Glu residue, a pH of 4.2 is required for a protonation probability of 50%, while in the case of the two Glu residues in close proximity, there is a higher attraction for protons, and therefore the pKa will be higher than 4.2.
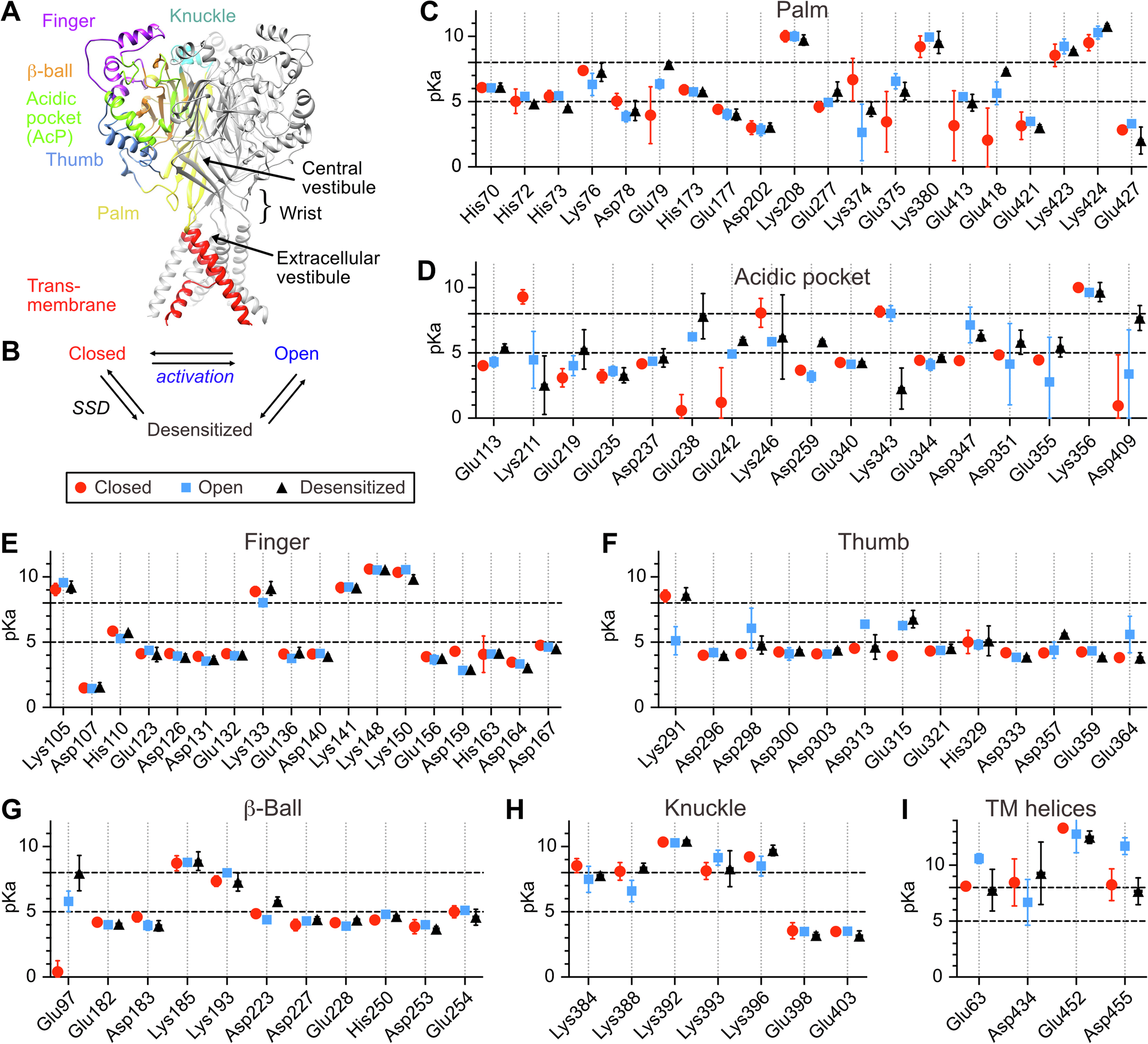
Based on the high-resolution structures of ASIC1a we have calculated the pKa values of titratable residues in ASIC1a by solving the Poisson-Boltzmann equation. This allowed us to rank the Asp, Glu His and Lys residues according to their potential as pH sensors. The 40 most highly ranked predicted pH sensing residues were individually mutated to a residue that retained most of their properties but disrupted titratability. The properties of these mutant channels were measured with electrophysiology. This showed that conservative mutations of 27 predicted pH sensors affected the pH dependence of the channel. Of most of these residues, mutations to amino acids with various properties were generated and functionally analyzed to determine which properties were important for the functional contribution of a given residue. Pairs of mutations were also generated to study the interaction between such putative proton-sensing residues. Our detailed analysis of these mutant channels allowed us to generate a map of pH-sensing residues of ASIC1a. It also highlighted hotspots of pH sensing, where several residues contribute together to pH sensing. The information of protonation changes obtained from the computational analysis allowed a rational interpretation of the functional effects of mutations.
With this map of protonation sites, we have uncovered the initial steps of ASIC activation. Some information on the subsequent steps, thus the conformational changes linking protonation to pore opening, are available from fluorescence analyses of conformation changes and from the comparison of closed and open ASIC structures. Additional information on these changes in conformation is however required and will likely be provided from fluorescence-based approaches and from molecular dynamics simulations. Together with such additional information, a more complete picture of the ASIC activation will be generated. Although several of the residues identified here were previously shown to affect the ASIC pH dependence in many different, isolated studies, their relative importance was not known. The present study provides a comprehensive view of the first step in ASIC activation. Confirmed protonation sites of ASIC1a are potential binding sites of interest for ASIC-targeting drugs. Besides providing a comprehensive map of pH sensing in the physiologically important ASIC1a, our study introduces a robust method for the investigation of pH sensing in other proteins.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in