Efficacy and tolerability of neoadjuvant therapy with Talimogene laherparepvec in cutaneous basal cell carcinoma: a phase II trial (NeoBCC trial)
Published in Cancer and Biomedical Research
https://www.nature.com/articles/s43018-024-00879-x
Cutaneous basal cell carcinoma (BCC) is the most common cancer worldwide with incidence rates ranging from 24 – 430 per 100 000 in Europe 1,2. The incidence has doubled over the last two decades and is expected to increase further 3,4.
A surgical approach is the treatment of choice for the majority of BCCs. However, in case of high-risk areas in the face and locally advanced tumors, they can be difficult-to-resect with requirement of plastic reconstructive surgery like skin flaps or grafts for wound closure. This entails the risk of detecting the local recurrence at a later time point 5 and can further result in a poor cosmetic outcome and lead to functional impairment, all of which can negatively impact the patient's well-being and quality of life 6.
Neoadjuvant treatment strategies aim to reduce post-surgical morbidity, increasing the likelihood of curative resection and reducing the risk of recurrence. We hypothesized that oncolytic viruses (OV), an innovative class of intratumoral immunotherapeutics, might be an effective and safe neoadjuvant treatment strategy for difficult-to-resect BCCs because: i) BCCs mainly grow locally on the skin and are easily accessible for intralesional injections, ii) BCCs carry a high tumor mutational burden (TMB) and abundant tumor neoantigens 7 and iii) BCCs have an immunosuppressive tumor microenvironment (TME) rich in regulatory T cells (Tregs) 8, which could be targeted by OV. The OV Talimogene laherparepvec (T-VEC) is a genetically engineered herpes simplex virus (HSV) 1 and is approved in Europe and the United States of America (US) for the treatment of unresectable, injectable lesions of stage IIIB-IVM1a melanoma 9.
Key findings
We performed a single arm, phase II neoadjuvant trial with T-VEC in 18 patients with difficult-to-resect cutaneous BCCs. The primary endpoint, defined as the proportion of patients, who after six cycles of T-VEC (13 weeks), become resectable without the need for plastic reconstructive surgery, was already achieved after stage I (9/18 patients; 50.0%), thus the study was discontinued for early success. The objective response rate was 55.6% and the complete pathological response rate was 33.3%. Secondary endpoints included safety, relapse free and overall survival, time to occurrence of new BCCs and biological read outs. Only mild adverse events occurred. The 6-months relapse free survival and overall survival rates were 100%. T-VEC led to a significant increase of cytotoxic T cells, B cells and myeloid cells, and a decrease of Tregs within the tumor microenvironment. We further detected hyperexpanded, cytotoxic T cell clones, and IgG1 plasma cell clones post-treatment.
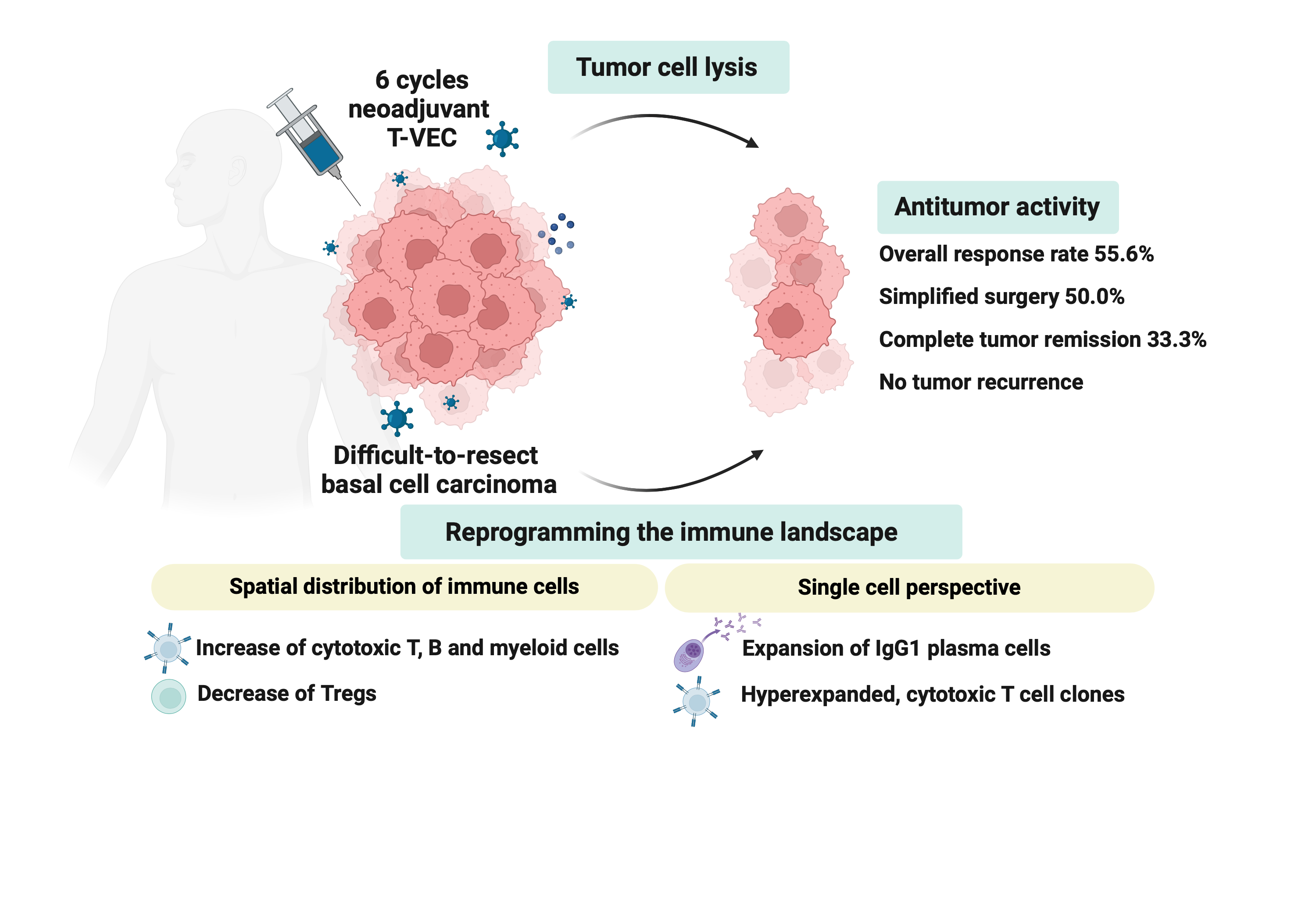
Graphical abstract of the NeoBCC trial. Six cycles of neoadjuvant talimogene laherparepvec (T-VEC) treatment in difficult-to-resect basal cell carcinoma, led to tumor cell lysis, reprogramming of the immune landscape and ultimately resulted in antitumor activity. The figure was created in BioRender. Ressler, J. (2021) BioRender.com/n44m000.
Future Directions
Our results warrant further investigation in randomized clinical trials as a stratified therapy and favors the development and investigation of next generation oncolytic virotherapies in the treatment of BCCs.
Collaborative Team Effort
This study was a true collaborative effort between clinical and preclinical research teams from the Medical University of Vienna, Department of Dermatology (Christoph Höller, Wolfgang Weninger, Matthias Farlik-Födinger) and the St. Anna Children's Cancer Research Institute (Florian Halbritter), as well as with the support of the team from the CCC Cancer Center and the dermatosurgical team of the Medical University of Vienna, Department of Dermatology. The project, led by the Medical University of Vienna, was supported by a research grant from Amgen. The first author of the study, Julia Maria Ressler, was able to devote herself to this project alongside her specialist training thanks to a Clinician Scientist Fellowship from the Austrian Society for Dermatology and Venereology.
References:
- Verkouteren, J.A.C., Ramdas, K.H.R., Wakkee, M. & Nijsten, T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol 177, 359-372 (2017).
- Kappelin, J., Green, A.C., Ingvar, Å., Ahnlide, I. & Nielsen, K. Incidence and trends of basal cell carcinoma in Sweden: a population-based registry study. Br J Dermatol 186, 963-969 (2022).
- Schreuder, K., Hollestein, L., Nijsten, T.E.C., Wakkee, M. & Louwman, M.W.J. A nationwide study of the incidence and trends of first and multiple basal cell carcinomas in the Netherlands and prediction of future incidence. Br J Dermatol 186, 476-484 (2022).
- El-Khalawany, M., et al. Epidemiological and clinicopathological analysis of basal cell carcinoma in Egyptian population: a 5-year retrospective multicenter study. J Cancer Res Clin Oncol (2022).
- Kondo, R.N., Gon, A.D.S. & Pontello Junior, R. Recurrence rate of basal cell carcinoma in patients submitted to skin flaps or grafts. An Bras Dermatol 94, 442-445 (2019).
- Răducu, L., et al. Quality of Life in Patients with Surgically Removed Skin Tumors. Medicina (Kaunas)56(2020).
- Jayaraman, S.S., Rayhan, D.J., Hazany, S. & Kolodney, M.S. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol 134, 213-220 (2014).
- Ressler, J.M., et al. Myofibroblast stroma differentiation in infiltrative basal cell carcinoma is accompanied by regulatory T-cells. J Cutan Pathol (2022).
- Andtbacka, R.H., et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 33, 2780-2788 (2015).
Follow the Topic
-
Nature Cancer

This journal aims to provide a unique forum through which the cancer community will learn about the latest, most significant cancer-related advances across the life, physical, applied and social sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in